Approach to the Patient with Pulmonary Nodules
INTRODUCTION
The radiographic finding of a pulmonary nodule, formerly known as a coin lesion, has long challenged the clinician. At the heart of the dilemma, the question remains unchanged: “Is it malignant or benign?” When faced with a pulmonary nodule, the clinician and the patient usually have one of three choices: (1) observe it with serial chest computed tomography (CT), (2) perform additional diagnostic tests (imaging and/or a biopsy), or (3) remove it surgically.
The proper choice depends on epidemiology, radiographic appearance, assessment of surgical risk, and patient preferences. For malignant lesions, early surgical resection still represents the best chance for cure. On the other hand, unnecessary resection of benign nodules exposes patients to the morbidity and mortality of a surgical procedure. The aim of this chapter is to review what is known about the pulmonary nodule to formulate a diagnostic approach to this often controversial problem. The goal will be to arrive at a systematic approach that will promptly identify and bring to surgery all patients with operable malignant nodules while avoiding thoracotomy in patients with benign nodules. To do this we need to have a clear definition of pulmonary nodules, information on their incidence and prevalence, the causes of malignant and benign nodules, the available imaging techniques, a method of estimating the probability of cancer, the strengths and weakness of serial CT imaging versus different biopsy techniques, and the impact of surgical risk and comorbidities on diagnostic strategies. These various considerations can then be integrated into an algorithm providing a unified approach to diagnosis and management.
DEFINITION
Pulmonary nodules should be characterized on the basis of number, size, and density as determined by CT. A solitary pulmonary nodule is defined as a single discrete pulmonary opacity that is surrounded by normal lung tissue that is not associated with adenopathy or atelectasis.1,2 Previously there was controversy as to what constituted the upper size limit for defining a solitary pulmonary nodule. Some early series included lesions up to 6 cm in size.3,4 However, it is now recognized that lesions larger than 3 cm are almost always malignant, so current convention is that solitary pulmonary nodules must be 3 cm or less in diameter.5,6 Larger lesions should be referred to as pulmonary masses and should be managed with the understanding that they are most likely malignant; prompt diagnosis and resection is usually advisable.7
The term “solitary pulmonary nodule” was originally used when most nodules were detected incidentally by chest radiography. Today, most nodules are detected by CT, which greatly enhances nodule detection and characterization. However, we now recognize that many nodules that would have been characterized previously as “solitary” by chest radiograph are actually not solitary, since there may be other small nodules present. Thus, the classical definition of pulmonary nodules needs to be revised to take into account data from more recent CT-based studies.1,7 As such, the term “solitary” should not be used for nodules accompanied by additional nodules or associated findings, or for nodules not completely surrounded by aerated lung.
CT has also increased awareness of subcentimeter nodules, which are defined as those ≤8 mm in diameter.1,8–10 Subcentimeter nodules may be spherical or nonspherical, and malignant nodules may have either shape.10 CT has also led to a more precise and nuanced classification of nodules according to their density. Nodules may have a pure solid appearance, a pure ground-glass appearance, or a mixed ground-glass and solid appearance. These characteristics can be used to help estimate the probability of cancer in the nodule.
INCIDENCE AND PREVALENCE
The incidence of pulmonary nodules and the probability of malignancy in those nodules vary widely, depending on the patient population; thus, many case series may not be directly comparable. This is a critical distinction when reviewing the literature. So the clinical context that led to nodule detection is a key factor to consider. Surgical case series, in which the denominator consists entirely of resected lung nodules, have a much higher prevalence of malignancy as compared to studies that use all nodules detected by chest radiograph or CT. Note that the prevalence of cancer seen in these surgical case series is not the same as the pretest probability of cancer for a newly identified lung nodule. Cancer prevalence in surgical series is high because the population being studied consists entirely of patients in whom the clinical suspicion of cancer based on prior testing has been deemed high enough to warrant the risk of surgery. Nodules unlikely to be malignant tend to be excluded from surgical case series. Given these limitations, surgical case series data can still be useful to gain insight into the factors that impact the probability of cancer, but the prevalence of cancer will be higher than in other clinical situations.
The frequency with which a pulmonary nodule is identified on chest radiography is on the order of 1 to 2 per 1000 chest radiographs.11 Most of these are clinically silent, and about 90% are noted as an incidental finding on radiographic examination. Younger patients from areas where granulomatous diseases such as tuberculosis, histoplasmosis, and coccidioidomycosis are endemic can be expected to have a lower malignancy rate. In an Air Force Medical Center study from Illinois of 137 patients, only 22 (16%) had a malignancy.12 Granulomas were diagnosed in 103 (75%) patients; 53 of them were attributable to histoplasmosis endemic to the area. Most of these patients (77%) were under age 45, and no malignant nodules were diagnosed in patients less than 35 years of age. This series predated the use of chest CT.
Importantly, the incidence of nodules and the probability of malignancy in the nodules are very different in more recent studies of low-dose CT screening for lung cancer among high-risk patients than in surgical case series. The prevalence of noncalcified nodules in observational studies of low-dose CT screening ranges from 13% to51%.13–15 Among nodules that are identified on the first screening study, the prevalence of cancer ranges from 3% to 12%.16 On follow-up CT imaging of the same population (i.e., incidence screens), the probability of new noncalcified nodules has varied widely, from 2.5% to 13%. The probability of cancer in these incidence nodules has varied from 5% to 23%.
MALIGNANT PULMONARY NODULES
Risk factors for malignancy have been identified from studies of pulmonary nodules and include patient age, smoking history, nodule size, and prior history of malignancy. Age is one of the most consistent risk factors. In a series of 370 resected indeterminate solitary pulmonary nodules, the incidence of malignancy increased from 63% for patients age 45 to 54 to 74% for ages 54 to 64 and continued to rise with age to 96% for those above the age of 75.17 Since this was a surgical case series, in which the denominator consists of resected lung nodules, the prevalence of malignancy is much higher as compared to studies of nodules detected by chest radiograph or during lung cancer screening, in which the denominator consists of all nodules detected. These findings correlate with those of previous studies, which also show that malignancy is very rarely found in patients under the age of 35.12,18,19
Smoking is closely correlated with the development of lung cancer, particularly squamous and small cell carcinoma. The Surgeon General’s report of 1964 and subsequent studies have demonstrated that the risk of lung cancer increases with the duration of smoking and the number of cigarettes smoked. Average smokers have about a 10-fold risk and heavy smokers a 20-fold risk of developing lung cancer when compared to nonsmokers. Smoking is responsible for about 85% of the cases of bronchogenic carcinoma. Cessation of smoking will reduce this risk after 10 to 20 years, but it now appears that former smokers have a slightly higher risk of cancer throughout their lifetimes.
Nodule size is closely correlated to risk of malignancy. Several series have demonstrated an increased incidence of malignancy with increasing nodule size. Nodules larger than 3 cm will be malignant 80% to 99% of the time. In seven studies of nodules detected in lung cancer screening trials, the prevalence of malignancy was 0% to 1% in patients with nodules <5 mm in diameter, 6% to 28% for 5- to 10-mm nodules, 33% to 64% for 11- to 20-mm nodules, and 64% to 82% for nodules measuring >20 mm.12,17,20–23
Primary bronchogenic carcinoma is the most common malignant tumor that presents as a pulmonary nodule.5,17,21,24 Histologically, adenocarcinoma and squamous cell carcinoma make up the majority; of the two, adenocarcinoma is the more common (Table 110-1). Of note, tumors that used to be called bronchioloalveolar cell carcinoma (BAC) have now been reclassified and the term adenocarcinoma in situ is used for BAC tumors once they have been confirmed by surgical resection. Small cell carcinoma presenting as a solitary pulmonary nodule is rare. Other rare primary lung tumors that may present as solitary pulmonary nodules are bronchial carcinoids, lymphomas, hemangioendotheliomas, and sarcomas.
Metastases may present as solitary pulmonary nodules in patients who have known primary malignancies or in whom the presence of primary malignancy is unknown. In up to 40% of such patients, who manifest only a single nodule on chest radiograph, CT scan may show other nodules that are not disclosed by plain chest radiograph.25,26 Even though a lesion is solitary, 33% to 95% of nodules in patients with an established diagnosis of cancer will be malignant.27–31 The most common histologic types of metastatic nodules are adenocarcinomas of the colon, breast, and kidney; head and neck tumors; sarcoma; and melanoma. Because of the high likelihood of malignancy, a nodule in a patient with an established diagnosis of cancer should be treated differently from other solitary nodules. Assuming no other obvious metastatic spread, one should consider proceeding directly to biopsy. Even in the presence of a known lung cancer, some of these nodules may represent a second primary pulmonary malignancy that is similar in histologic appearance. Immunohistochemistry and other confirmatory marker studies may be indicated to determine the nature of the nodule. A solitary pulmonary nodule in a patient with a history of malignant disease should be resected as long as there is no other evidence of recurrent or metastatic disease.
BENIGN PULMONARY NODULES
Benign solitary pulmonary nodules are more common in the young and in nonsmokers. Causes include benign tumors such as hamartomas, both infectious and noninfectious granulomas, vascular lesions, and rare miscellaneous conditions (Table 110-1).
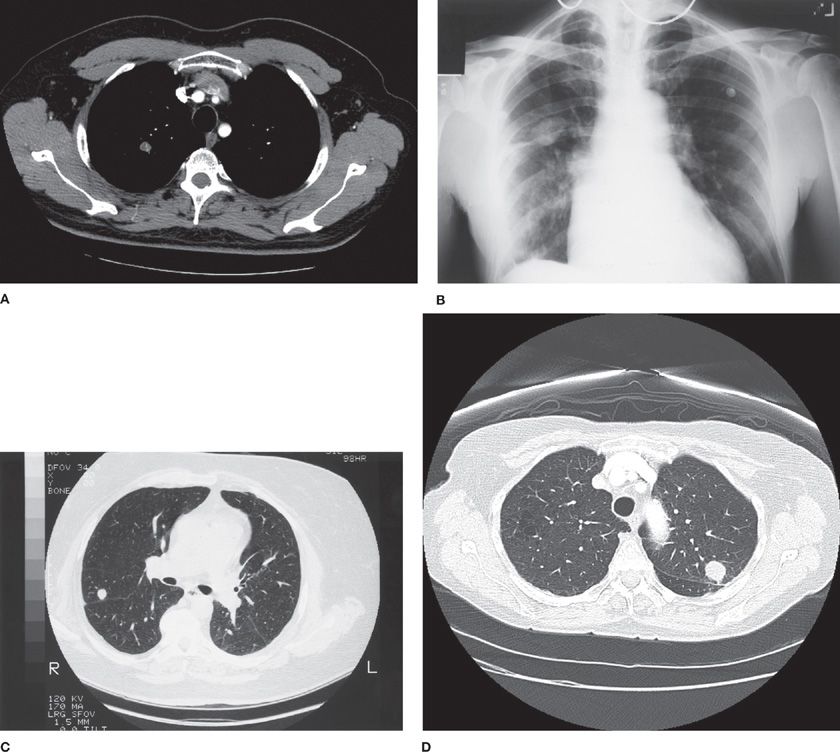
Hamartomas are the most common benign tumors presenting as solitary pulmonary nodules. They are believed to be developmental malformations composed mainly of cartilage, fibromyxoid stroma, and adipose tissue. A review of six series with 3802 resected solitary pulmonary nodules found that 5% were hamartomas.12,17,19,32–34 In a series of 215 hamartomas resected at the Mayo Clinic, the peak incidence was in the seventh decade of life; male-to-female ratio was 1:1; and the average size was 1.5 cm, although some were as big as 6 cm.35 Most hamartomas were asymptomatic (97%), and 17% were noted to grow slowly on serial radiographic examination. They may be identified radiographically by a pattern of “popcorn” calcification, which is often intermixed with areas of low attenuation on CT scan representing fat deposits within the nodule. CT appearance will be diagnostic in about 50% of hamartomas (Fig. 110-1).36
Figure 110-1 Benign pulmonary nodules and their radiographic patterns: A. Hamartoma with fat density within the nodule. B. Pseudotumor due to fluid in a fissure, the result of both pleural disease and fluid overload, has the appearance of a pulmonary mass. C. Noncontrast CT shows a round 1-cm nodule, with relatively high radiographic density, proven on resection to be a granuloma. D. Nodule with smooth border, diagnosis histoplasmosis.
Infectious granulomas make up more than 90% of all benign nodules. They arise as a result of healing after infection from a variety of organisms. The offending agents will vary, depending on geographic location. Among the most common causes are histoplasmosis, coccidioidomycosis, and tuberculosis. Other, less common causes are dirofilariasis (dog heartworm), mycetoma, echinococcal cyst, and ascariasis. A history of exposure is important in establishing a possible infectious origin. Clues such as tuberculosis exposure history and skin test results, prior travel history, places of residence, occupation, and pets may be invaluable in some instances.
A variety of inflammatory diseases can also cause benign pulmonary nodules. Noninfectious granulomas sometimes present as pulmonary nodules in systemic diseases such as sarcoidosis, in which nodules are not invariably accompanied by hilar adenopathy. Rheumatoid arthritis may also be associated with pulmonary nodules, usually in patients with active rheumatoid disease who will also have subcutaneous nodules. Granulomatosis with polyangiitis (formerly Wegener’s granulomatosis) can also present with pulmonary nodules, which are often cavitary. In such cases there are usually multiple nodules and there may be concurrent renal disease.
Miscellaneous causes of benign pulmonary nodules have been described. Some of the more common conditions are lung abscess; rounded or spherical pneumonia; pseudotumor, which represents fluid in an interlobar fissure (Fig. 110-1); hematomas after thoracic trauma or surgery; and fibrosis or scars resulting from the resolution of infectious or inflammatory process. Rarer conditions presenting as pulmonary nodules include silicosis, bronchogenic cyst, amyloidosis, pulmonary infarct, and vascular anomalies. Arteriovenous malformations may also present as pulmonary nodules. They may grow slowly, and have a characteristic appearance on contrast-enhanced CT scan, with identification of afferent and efferent vessels emanating from and heading toward the hilum, respectively.
IMAGING TECHNIQUES
Imaging techniques are often helpful in distinguishing benign from malignant causes of pulmonary nodules, and as such they play a key role in their evaluation and management. During the last decade, rapid advances in both CT and positron emission tomography (PET) have dramatically changed the diagnostic approach to pulmonary nodules. However, this does not mean that these techniques should be used indiscriminately. Cost-effective strategies to manage pulmonary nodules require that we understand the performance characteristics (sensitivity, specificity), strengths, and weaknesses of each of these technologies, so that they can be applied properly.37 The primary technologies that need to be considered are plain chest radiography, CT, and PET.
 PLAIN CHEST RADIOGRAPHY
PLAIN CHEST RADIOGRAPHY
Many pulmonary nodules are discovered on routine plain chest radiograph while asymptomatic. Nodules are usually identifiable on chest radiograph by the time they are 0.8 to 1 cm in diameter, although nodules 0.5 to 0.6 cm can occasionally be seen.11 Most will be identified on posteroanterior (PA) projection, but some will be seen only on lateral projection, so standard PA and lateral chest radiography should be obtained whenever possible. When a nodule can be seen only on one projection, the clinician should question whether it is truly in the lung parenchyma. Structures overlying the lungs, such as leads used for cardiac monitoring, nipple shadows, skin lesions, and rib lesions can all mimic a pulmonary nodule, as can pulmonary vessels viewed end on. If in doubt, a CT should be done. Once it has been ascertained that a true nodule exists, the first step is to make every effort to obtain previous radiographs for comparison. A solid nodule that has remained stable with no increase in size for 2 years by CT is very likely benign and warrants no further investigation. Conversely, a nodule that was not present on a comparable radiograph within the past 2 months is unlikely to be malignant, having grown so rapidly.
 COMPUTED TOMOGRAPHY
COMPUTED TOMOGRAPHY
Chest CT should be part of the evaluation of all lung nodules, either alone or as part of a combined PET-CT. As with chest radiography, comparison with old films is critical, since it can provide an estimate of the growth rate over time. CT can pinpoint the exact location of the nodule and provide three-dimensional images of the lesion. Thin-section high-resolution CT can better define the borders and the nodule’s relation to adjacent structures, such as vessels and the pleura.23,38 CT is also more sensitive than standard chest radiographs in detecting calcifications and can quantify calcification in nodules even when they are not readily visible to the naked eye.23,39 Nodules with higher radiographic density are more likely to be benign (Fig. 110-1).
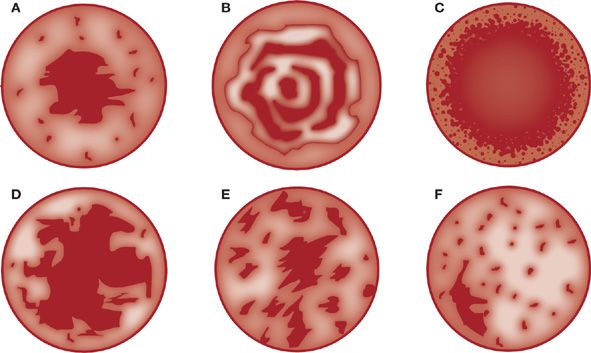
Calcification is generally an indication of benignity in a pulmonary nodule. Infectious granulomas tend to calcify with central, diffuse, or stippled patterns (Fig. 110-2). Laminar or concentric calcification is characteristic of granulomas caused by histoplasmosis. A popcorn calcification pattern is typically seen in hamartomas and when coupled with fat density within the same nodule is highly specific. Eccentric calcification patterns should make one suspicious for malignancy. It should be noted that, in general, 6% to 14% of malignant nodules exhibit calcification. When calcifications are present in malignant lesions, they are usually eccentric and few. Benign patterns of calcification (central, diffuse, laminar, or popcorn) are very rare in malignant nodules. In one study of 1267 solitary pulmonary nodules, only seven malignant nodules (0.6%) had a benign calcification pattern.40 Most nodules with a benign calcification pattern can be observed with serial CT scans.
Figure 110-2 Patterns of calcification in nodules. A. Central. B. Laminated. C. Diffuse. D. Popcorn. E. Stippled. F. Eccentric. Patterns A, B, C, and D generally indicate a benign process; E and F suggest malignancy. (Data from Lillington GA. Management of solitary pulmonary nodules. Dis Mon. 1991;37(5):271–318.)
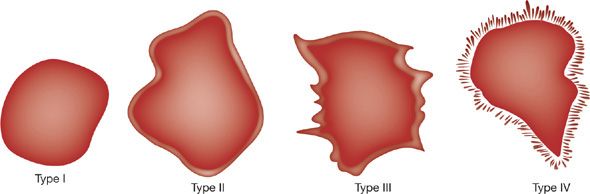
The edge characteristics of nodules can also offer insight into whether or not a lesion is malignant. Benign lesions are often well circumscribed with a round appearance whereas malignant nodules tend to have irregular or lobulated borders (Fig. 110-3). CT imaging characteristics that suggest malignancy include spiculated margins (likelihood ratio [LR] 5.5),23,24,38 pleural retraction (LR 1.9), and a vessel sign (LR 1.7). The feeding vessel sign consists of a vessel leading directly to a nodule. This is often associated with septic embolism but also occurs with metastasis, arteriovenous fistulas, and rarely in lung cancer. Lobulated margins (LR 1.1) are associated with malignancy but the predictive value is low.41 Other patterns that have been reported to be associated with malignancy include vascular convergence (which suggests vascular and/or lymphatic invasion),42 a dilated bronchus leading into the nodule,43 pseudocavitation (“bubbly” appearance thought to represent air bronchiolograms),38 and true cavitation when it is associated with a thick and irregular wall.44 However, none of these radiographic signs is entirely specific for malignancy. Malignancy is somewhat less likely if a bronchus sign is present although its predictive value is low. An air-bronchus sign can be seen in primary pulmonary lymphoma, but this is a very rare disease, accounting for less than 1% of all lung cancers. Malignancy is much less likely if a nodule has smooth or polygonal margins (LR 0.2).
Figure 110-3 Characteristic appearance of nodule edges. Type I is sharp and smooth, type II is lobulated, type III has irregular undulations, and type IV is grossly irregular with many spiculations. (Data from Siegelman SS, Khouri NF, Leo FP, Fishman EK, Braverman RM, Zerhouni EA. Solitary pulmonary nodules: CT assessment. Radiology. 1986;160(2):307–312.)
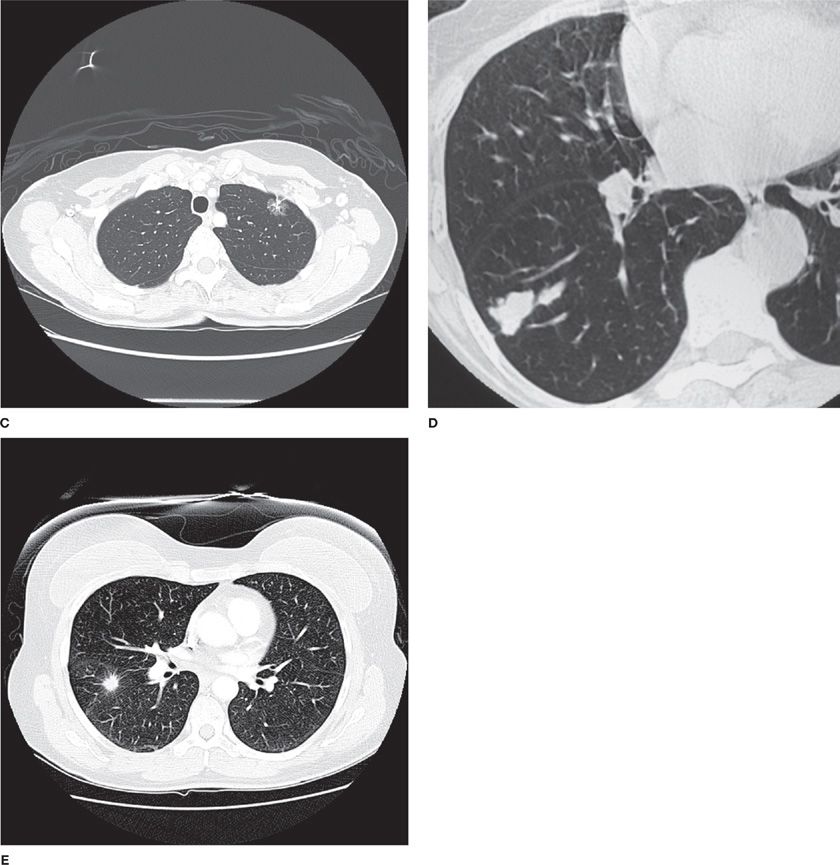
Rapid advances in CT technology have also led to more precise characterization of the density of lung nodules. It is now appreciated that nodules may be characterized as solid, partly solid, or pure ground-glass opacities (defined as focal densities in which underlying lung morphology is preserved) (Fig. 110-4). This is particularly useful for categorizing small nodules (<1 cm) since these categories can help to distinguish benign from malignant nodules. The percentage of pure ground-glass opacities that are malignant varies significantly in the literature, from 18% to almost 60%.45–47 For subcentimeter nodules, the likelihood of malignancy is similarly high in partly solid lesions, but much lower (<10%) in solid nodules.45,47
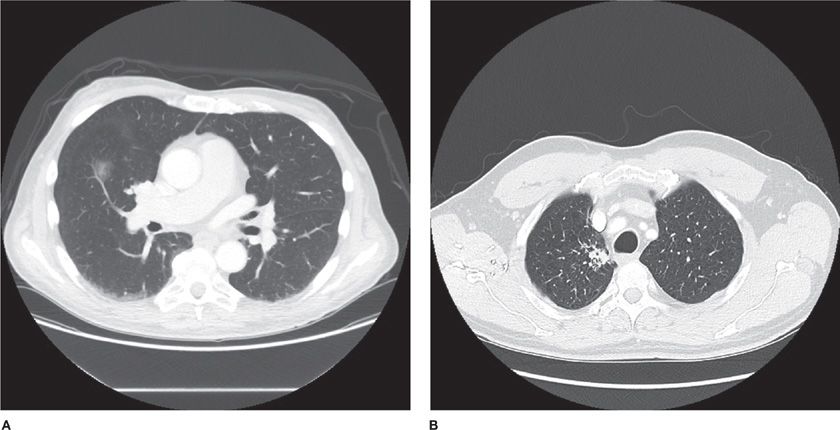
Figure 110-4 Malignant nodules with different densities and borders: A. Ground-glass nodule, right middle lobe, diagnosis adenocarcinoma in situ. B. Air bronchogram with semisolid nodule, diagnosis adenocarcinoma. C. Semisolid nodule D. Solid lung nodule, lobulated margin. E. Solid lung nodule, spiculated margin.
Ground-glass nodules may represent either atypical adenomatous hyperplasia (AAH) or adenocarcinoma in situ (formerly BAC).42,48–55 In contrast, partly solid or solid nodules usually represent adenocarcinoma, but can also be caused by squamous cell carcinoma or small cell carcinoma. When pure ground-glass opacities start to grow and become more solid, demonstrating a replacement growth pattern, this should be considered highly suspicious for adenocarcinoma.1,56,57 Of note, observed growth rates are often very slow for malignant ground-glass opacities, intermediate for partly solid nodules, and relatively fast for solid nodules.58
The superior resolution of multidetector scanners has also facilitated the development of volumetric CT. Volumetric CT may allow growing lesions to be identified earlier than conventional transverse CT. Preliminary studies demonstrated that three-dimensional volume analysis enabled tumor growth to be detected in 5-mm diameter nodules as early as 30 days after the initial CT.59,60 Volumetric CT was successfully used to guide evaluation of small lung nodules in the NELSON trial.8,61 In that trial, the investigators used serial volumetric CT measurements to calculate the doubling time of indeterminate lung nodules. CT volume doubling time of <400 days or a new solid component in a previously nonsolid nodule was defined as positive.8 Follow-up imaging could detect growth as early as 6 weeks after imaging of an indeterminate nodule. The second round screening in this study had a sensitivity of 96.4%, specificity of 99.0%, positive predictive value of 42.2%, and a negative predictive value of 99.9%.
A complementary technique is dynamic CT, which uses iodinated IV contrast to look for enhancement of nodules.62 Although malignant nodules enhance more than benign ones, benign lesions, such as hamartomas and tuberculomas, may also enhance. Median sensitivity and specificity in seven studies from a systematic review were 96% and 75%, respectively.20,62–68 In a multicenter trial using dynamic CT the negative predictive value was 97%. However, lack of specificity has limited the utility of this test, since it cannot reliably distinguish between malignant and active inflammatory or infectious nodules.2 As a result few centers at the present time are using this approach.
 POSITRON EMISSION TOMOGRAPHY
POSITRON EMISSION TOMOGRAPHY
PET can be used to help differentiate noninvasively between malignant and benign nodules. PET takes advantage of the fact that tumor cells have increased glucose uptake and metabolism. A D-glucose analog labeled with a positron-emitting fluorine-18 radioisotope (FDG) is injected into the patient, and uptake by the nodule is then measured. Malignant nodules have a higher uptake of FDG. Sensitivity of PET in systematic reviews has ranged from 87% to 95%.2,20,69 Integrated PET-CT scanners combine the CT and FDG imaging gantry, allowing more precise anatomic localization of areas of FDG uptake than dedicated PET imaging alone.70–72 PET imaging also provides useful information regarding lung cancer staging, since it will occasionally detect unsuspected distant metastases.73
It is important to recognize that most studies of PET have used a single threshold level for discriminating malignant from benign nodules, typically a standard uptake value (SUV) of 2.5.2,73 However, PET images actually provide a range of possible results, depending on the amount of uptake in the nodule as compared to that of the mediastinal blood pool. In a study of 344 patients undergoing PET-CT, the likelihood ratios (LRs) for benign, probably benign, indeterminate, probably malignant, and definitely malignant were 0.03, 0.15, 1.01, 3.2, and 9.9, respectively.74 When FDG uptake was slightly greater than the mediastinal blood pool, this was considered as probably malignant. Substantially greater uptake was considered as definitely malignant. Indeterminate readings were rare (1%). This study suggests that greater FDG uptake is more strongly associated with malignancy. Clinically, the implication is that physicians should consider not only whether a PET scan is positive, but should also consider the degree of FDG uptake when estimating the posterior probability of disease after PET imaging.
However, PET appears to be less sensitive for lesions less than 8 mm in size, so its use should be limited to those lesions ≥8 mm in size.2,75 While there is limited preliminary evidence that PET may be useful for lesions as small as 8 to 10 mm in size, there are still too many false negatives reported to make PET useful for smaller lesions outside of a clinical trial at the current time.76–78 False-negative findings have also been seen in patients with lepidic predominant adenocarcinomas (formerly BAC, also minimally invasive or adenocarcinoma in situ), carcinoids, and mucinous adenocarcinomas.78,79 False positives have been seen in patients with granulomatous infections, such as tuberculosis, atypical mycobacterial disease, and endemic mycoses, as well as in patients with inflammatory conditions, such as rheumatoid arthritis and sarcoidosis.80,81 Theoretically, false-positive results can also be caused by uncontrolled hyperglycemia.82
DISTINGUISHING BETWEEN BENIGN AND MALIGNANT NODULES
 ESTIMATING PROBABILITY OF MALIGNANCY
ESTIMATING PROBABILITY OF MALIGNANCY
Epidemiology, knowledge of the different causes of malignant and benign nodules, and the imaging characteristics of the nodule all serve to inform the physician as to the probability of malignancy in a given nodule. While physicians often estimate pretest probability of cancer intuitively, several investigators have attempted to develop mathematical models to estimate the probability of malignancy of indeterminate pulmonary nodules.83–86 Using clinical and radiographic characteristics of malignancy derived from the literature, these authors have analyzed some combination of the following risk factors by Bayesian, neural network, and other methods to obtain a mathematical estimate of the probability of malignancy: nodule size, location, growth rate, margin characteristics, age of the patient, smoking history, prevalence of malignancy in the community, and occult calcification on CT densitometry.83–85,87–90
For example, in the Bayesian approach, each risk factor for a particular patient and nodule is assigned a LR of malignancy derived from published data. In one model, overall prevalence of malignancy, diameter of the nodule, patient’s age, and smoking history were considered.84 The LRs for malignancy of each of these factors were then multiplied to provide odds of malignancy, which are then converted into a percent probability of cancer. In a computerized neural network model that utilizes nonlinear mathematics to analyze input data, risk factors for malignancy were used and compared to the results of Bayesian analysis.85 The authors found that their neural network was not as accurate as Bayesian analysis in predicting malignancy.
One of the problems with these and other methods is the quality of the input data (i.e., the LRs), which may not be representative of all patient populations. In addition, Bayesian analysis presupposes that the LRs for a particular risk factor are not affected by the presence or absence of any other factor. It is not clear that this is true of the LRs. Therefore, although mathematical models to predict probability of malignancy may seem attractive, the complexity of the issue once again leaves us with an uncertain answer. This may explain why the previously described methods are not in widespread clinical use. It is worth noting that the accuracy of models for predicting malignancy appears to be similar to that of expert physicians, although the correlation is poor, which suggests that models may provide additional insights.87
However, assessment of the pretest probability of malignancy is central to optimal strategy selection when managing pulmonary nodules.1,2,37,56,91 While these formulas and neural networks may lack precision on an individual patient level, they can serve to inform decision making as to what risk factors to pay attention to and how important they are relative to each other.5 Risk factors associated with a low probability of malignancy include diameter less than 1.5 cm, age less than 45 years, absence of tobacco use, having quit for 7 or more years, and a smooth appearance on radiographic imaging. Risk factors associated with a moderately increased risk of malignancy include diameter 1.5 to 2.2 cm, age 45 to 59, smoking up to 20 cigarettes per day or being a former smoker within the last 7 years, and a scalloped edge appearance on radiography. Risk factors associated with a high risk of malignancy include a diameter of 2.3 cm or greater, age greater than 60 years, being a current smoker of more than 20 cigarettes per day, a history of prior cancer, and a corona radiata or spiculated appearance on radiography.4,5,87,92–94
After consideration of these risk factors and possibly using one of the validated models to arrive at an estimate of the pretest probability of malignancy, physicians must then choose between alternative strategies. The main alternatives are (1) careful observation with serial CT imaging; (2) nonsurgical biopsy using CT guidance or bronchoscopy; or (3) surgery. The results of each strategy help to further refine the probability of malignancy, either up or down. As such, it is important to consider the strengths and weaknesses of each of these approaches.
 CAREFUL OBSERVATION WITH SERIAL CT IMAGING
CAREFUL OBSERVATION WITH SERIAL CT IMAGING
Careful observation with serial CT is predicated on the ability of the imaging technology to detect nodule growth. The fundamental assumption is that by comparing serial images over time a nodule’s growth rate can be determined and this in turn can be used to help distinguish between benign and malignant nodules. Squamous and large cell tumors have an average doubling time (i.e., the time for a nodule to double in volume) of 60 to 80 days. Adenocarcinomas double at about 120 days, and the rare small cell carcinoma that presents as a solitary pulmonary nodule can have a doubling time of less than 30 days.27 A nodule that has doubled in weeks to months is probably malignant and should be removed when possible.5 Benign nodules have doubling times of less than 20 days or more than 400 days. A nodule that doubles in size in less than 20 days is usually the result of an acute infectious or inflammatory process, while those that grow very slowly are usually chronic granulomatous reactions or hamartomas. Nodules with doubling times over 400 days can be observed with serial radiographs.1,2,5 This is the basis for the clinical axiom that 2-year radiographic stability is strong presumptive evidence that a nodule is benign.
It should be noted that controversy remains regarding how long follow-up should be continued. While traditional teaching has recommended observing lesions for a maximum of 2 years, it is now recognized that for some lesions longer follow-up may be warranted. Long doubling times have been observed in malignant lesions that presented as ground-glass nodules or as partially solid nodules.48,54,58 As a consequence, longer follow-up extending over years may be appropriate in patients with pure ground-glass nodules, especially if there is an antecedent history of lung cancer.95 However, for most solid nodules, 2 years of follow-up without evidence of growth is sufficiently long to warrant discontinuation of CT imaging.
Determination of nodule growth is based on the assumption that nodules are more or less spherical. Growth of a sphere must be considered in three-dimensional volume, not in two-dimensional diameter. The formula for volume of a sphere is 4/3(π)r3, or 1/6(π)d3, where r = radius and d = diameter. A nodule originally 1 cm in diameter whose diameter is now 1.3 cm has actually more than doubled in volume. Similarly, a 2-cm nodule has doubled in volume by the time its diameter reaches 2.5 cm. A nodule that has doubled in diameter has undergone an eightfold increase in volume. When old radiographs are available, growth rate and nodule doubling time can be estimated. If the diameter of a nodule is measured at two different points in time (first t1 and later at t2), then doubling time in days = (t × log 2)/[3 × log (d2/d1)]. In this formula t is the number of days between t2 and t1; d1 and d2 are the diameters of the nodule at times t1 and t2, respectively. It is therefore critical that old radiographs and CT scans be obtained for comparison when evaluating pulmonary nodules, since they can provide valuable insights into the doubling time and natural history of the nodule.
Accepting the assumption that a tumor arises from serial doublings of a single cancerous cell, we can estimate that it will take 27 doublings for it to reach 0.5 cm. By the time a nodule is 1 cm in diameter, it represents 30 doubling times and about 1 billion tumor cells. Depending on the exact growth rate, this theoretical 1-cm nodule has probably existed for years before it is detected, as malignant bronchogenic tumors have doubling times estimated at between 20 and 400 days. The natural history of a tumor usually spans about 40 doublings, whereupon the tumor is 10 cm in diameter and the patient has usually died.96
Nodule growth rate and doubling times become clinically relevant when deciding how often to order follow-up imaging when observing a pulmonary nodule. The question often arises whether observing a pulmonary nodule for an extra 3 to 6 months increases the likelihood of metastatic disease, since that nodule has probably been growing for years. There is no convincing empiric evidence to support this hypothesis. Whether delays longer than 3 to 6 months are safe is unknown. However, estimating this hazard of delay is clinically relevant, since the optimal frequency of serial CT follow-up imaging to monitor nodules for growth is predicated on limiting this hazard of delay. The question is, how frequently do follow-up scans need to be done to minimize the hazard of delay while containing costs and avoiding excessive radiation exposure?
Traditional practice, based on little empiric evidence, recommended that when a careful observation strategy was warranted, repeat CT scans be done at 3, 6, 12, and 24 months.5 However, more recent data from lung cancer screening trials using CT imaging suggests that a less aggressive practice may be reasonable in some patients with very small nodules.1,2,8,92,97–99 Therefore, decisions about the frequency and duration of follow-up for patients with pulmonary nodules need to consider multiple dimensions of the problem, including clinical risk factors, nodule size, radiation dose, surgical risks, patient preferences, cost, and the limits of imaging technology resolution (especially at sizes less than 5 mm).100–102 All of these can affect the optimal frequency of CT follow-up.
The ability to detect nodule growth is a function of image resolution. CT imaging greatly improved the ability to detect nodule growth as compared to conventional chest radiographs. This has made the careful observation strategy with serial imaging more effective, since it limits the hazard of delay by allowing earlier detection of growth. However, the accuracy of size measurements is still a problem when nodules are small. There is poor inter- and intraobserver variability when size differences of <1.5 mm are being assessed with two-dimensional (2D) CT.103,104 As a result, 2D measurements of small nodules may not always be reliable. The presence of growth was incorrectly assessed in 27% to 37% of CT scan pairs when diameters or cross-sectional areas were used as compared to a reference standard of volumetric measurement.2,101,105,106 Limited data suggests that volumetric measurements may provide a better alternative. When evaluated retrospectively, volumetric measurements using two CT scans performed a median of 3.7 months apart with a threshold volume doubling time >500 days was found to be 91% sensitive and 90% specific for malignancy. Another small study of predominantly solid nodules found that volumetric imaging changed management strategy in 10% of cases.107 Volumetric imaging and doubling times were also used successfully to guide management in the NELSON lung cancer screening trial.8 Unfortunately, volumetric measurement is time consuming and labor intensive and therefore has not yet become the standard of care.
Given this framework, it is reasonable to apply more recent expert consensus-based guidelines to help guide the frequency of follow-up CT imaging for the pulmonary nodule.2,95,108 For follow-up studies, imaging should be performed without contrast with thin sections using low-dose techniques.2 Where available, volumetric CT may further facilitate growth detection and limit the hazard of delay. In all cases patients should be informed about the potential benefits and harms including the hazard of delay versus the risks of unnecessary testing. The frequency and duration of CT imaging for solid nodules in patients who are potentially treatable is shown in Table 110-2. For patients with indeterminate pure ground-glass nodules and patients with part-solid nodules the frequency and duration of surveillance is different (Table 110-3).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree