Approach to the Patient with Cardiac Arrhythmias: Introduction
The diagnosis and management of specific cardiac arrhythmias are detailed in other chapters in this textbook. This chapter provides the clinician with an approach to the overall evaluation of patients presumed to have a cardiac arrhythmia. Without a doubt, the two key elements in assessing patients are the history and, if available, the electrocardiogram (ECG) rhythm strip obtained at the time of their symptom. Findings on the physical examination and judicious use of noninvasive and invasive tests can be quite helpful in certain circumstances.
History
It is imperative that a complete history of the patient’s symptoms be obtained. Many elements must be sought for in this process, including (1) documentation of initial onset of symptoms; (2) complete characterization of symptoms; (3) identifying conditions that appear to initiate symptoms; (4) duration of episodes; (5) frequency of episodes; (6) pattern of symptoms over time, for example, better or worse; (7) effect of any treatment; and (8) family history of a similar problem. It is also important to ascertain any pertinent past medical history that might be helpful in the diagnosis. This might include history of myocardial infarction (MI), especially in a patient who presents with palpitations and syncope, or the recent initiation of a drug that can cause hypotension, for example, an antihypertensive agent, in a patient who now presents with dizzy spells. In our experience, careful and thorough attention to obtaining the preceding information typically results in an efficient and focused approach to the patient’s problem.
In patients with supraventricular tachycardia with a 1:1 atrioventricular (AV) conduction pattern, the differential diagnosis is typically between AV reentry (AVRT), AV node reentry (AVNRT), and atrial tachycardia (AT). Thorough history taking can often lead one to the correct diagnosis even in the absence of an ECG tracing. For example, the onset of tachycardia with bending over or squatting is often present with AVRT or AVNRT, but not with AT. Palpitations associated with or aggravated by caffeine are more likely due to sinus tachycardia than an arrhythmia. In the differential diagnosis between AVRT and AVNRT, onset of symptoms in a woman >50 years old clearly favors AVNRT as the diagnosis. In fact, a recent report analyzing clinical variables in paroxysmal supraventricular tachycardia (PSVT) concluded that older age of onset, female sex, and presence of neck palpitations during tachycardia supported the diagnosis of AVNRT.1 We have also noted that the rate of the spontaneous tachycardia contains diagnostic power in adults, and rates of ≥250 beats/min are almost always AVNRT and not AT or AVRT.
Physical Examination
Observations from the physical examination are helpful primarily to define whether cardiovascular disease is present. For example, in a patient who presents with dizzy spells or syncope, the presence of orthostatic hypotension should alert the clinician to investigate whether this is the cause of the clinical symptoms. However, presence of a carotid bruit or decreased peripheral pulses may be important findings related to atherosclerosis that lead to a workup of coronary artery disease. Most importantly, the presence of specific cardiac murmurs or an S3 or S4 gallop may direct the clinician toward a cardiac cause for the patient’s symptoms. Also pay attention to the patient’s sex and age. PSVT that occurs in a 7-year-old boy is more likely caused by AVRT, whereas PSVT presenting in a 65-year-old woman more commonly is caused by AVNRT.
Syncope, Presyncope, and Dizziness
Patients with syncope, presyncope, or dizziness are often referred to the electrophysiologist for evaluation for fear that the symptoms are caused by an arrhythmia. Unless an ECG rhythm strip is recorded at the time of the patient’s event, it is impossible to eliminate positively an arrhythmic cause. Regardless, a detailed history typically points in the correct direction (see Chap. 48). The ECG may disclose many clues to the cause of syncope, including MI, cardiac hypertrophy, sinus node dysfunction, conduction abnormality, Wolff-Parkinson-White syndrome, long or short QT interval, or Brugada syndrome. Evaluation of the echocardiogram may lead to a variety of cardiac diagnoses.
Neurally mediated syncope is very common. Typically, the patient is in an upright position, either sitting or standing, and may recount a feeling of being hot or warm with or without concomitant nausea prior to loss of consciousness. Sweating is a common feature, but the patient may state that it occurred on regaining consciousness rather than prior to syncope. Normally, the patient is alert on regaining consciousness but may feel fatigued. It is important to ask the patient if any bystander present mentioned how the patient looked after the event—comments that the patient was “pale as a ghost” or “white as a sheet” are typical in these circumstances. Although patients often state that their heart was pounding or faster than usual on awakening, they do not give a history of a rapid regular pulse that persists for minutes after the event. This latter feature should direct one to a possible arrhythmic cause for syncope. Church syncope, that is, patients who have presyncope or syncope during church services, is almost always vagally mediated in our experience.
Cardiac syncope is often sudden in onset and frequently unaccompanied by any prodrome. In some circumstances, patients relate a feeling of rapid palpitations prior to loss of consciousness, and these individuals should be evaluated for a cardiac arrhythmia regardless of whether heart disease is present. One should remember that rapid PSVT as well as ventricular tachycardia can cause syncope. Unfortunately, a sudden loss of consciousness without prodrome is not specific for an arrhythmia, and patients with an arrhythmia can present with some features of a vasovagal syncope.
For patients who present with dizziness or presyncope, it is important to distinguish between vertigo and true light-headedness. Ask patients whether they feel like the room is spinning or they are spinning, compared with a sensation that the lights are going out or they are about to lose consciousness, especially if they are taking medication. Additional tests depend on the results of the initial workup as described earlier.
Palpitations
Palpitations are described by patients in many ways including skipped beats, a sudden thump, hard beating, fluttering in the chest, a jittery sensation, a rapid pulse, or as merely a vague feeling that their heart is irregular. The authors have noted that many patients equate a strong heartbeat as palpitations, and it is important to distinguish this from irregular heartbeats. A premature atrial or ventricular complex often cannot be felt by the patient, and what they experience is the strong heartbeat that follows the pause. It may be useful to tap out various cadences for the patient. For example, to distinguish between atrial fibrillation (AF) and PSVT, tap out a rapid, irregular cadence compared with a rapid regular cadence—patients often recognize one over the other. Similarly, tap out a cadence of extra beats with a pause. Palpitations are often more prominent at night, especially when patients lie on their left side. Although these may be premature beats, often it is simply sinus rhythm. A feeling of cough or shortness of breath with extra beats is more typical of premature ventricular than atrial complexes.
Other historic features often tailor the initial workup. A rapid regular rhythm that occurs a few times per year and has been ongoing for many years is likely a form of PSVT. In the absence of a previous correlation with an ECG rhythm strip or 12-lead ECG, an electrophysiologic study typically is required for diagnostic and/or therapeutic reasons. Noninvasive monitoring for such infrequent arrhythmias is usually futile, and an electrophysiologic study is preferred over prescription of an implantable loop recorder. In contrast, for patients with more frequent symptoms, noninvasive event recorder monitoring is often our choice, and a unique new form of technology using wireless outpatient continuous monitoring can even identify asymptomatic arrhythmic episodes.2 Even if sinus rhythm is identified as the cause of palpitations, the results are valuable to the patient. Most patients want a diagnosis and are reassured that their symptoms are not life threatening, often the reason that brought them to the physician.
Women might present with palpitations during the week prior to menstruation, and the diagnosis is often premature atrial or ventricular complexes that occur during a specific time of hormonal change. It is commonly believed that alcohol and caffeine are arrhythmogenic; and although this may be so in certain patients, it has been our experience that these agents typically play a minor role in the majority of patients who have arrhythmias. Obviously, patients with AF might have episodes during alcohol intake, but such is usually not the case for PSVT and sustained ventricular tachycardia.
Fatigue, Chest Pain, and Dyspnea
On occasion, patients present with symptoms such as fatigue, chest pain, or dyspnea that seem unrelated to an arrhythmia. This is particularly true for those who have AF. It is surprising how many patients with AF do not experience palpitations and present with either fatigue or shortness of breath. Thus, although these symptoms typically direct the clinician down another diagnostic road, remember that they might be caused by an arrhythmia. Of particular importance are patients who present with AF and symptoms of heart failure without palpitations. Often these individuals have tachycardia-mediated cardiomyopathy, and with appropriate control of the ventricular rate, the ventricular function might even normalize. Because the patients do not experience palpitations, they typically present with symptoms of myocardial dysfunction secondary to the prolonged rapid ventricular rates experienced during AF.
Adjunctive Tests
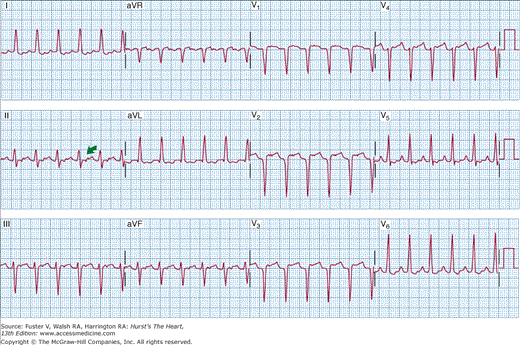
The ECG is covered elsewhere (see Chap. 15), but two specific findings on the 12-lead ECG during PSVT (Figs. 39–1 and 39–2) should be emphasized. Figure 39–1 demonstrates a pseudo r′ in ECG lead V1 that is typical for patients who present with AV node reentry. The pseudo r′ results from superimposition of the P wave on the end of the QRS complex and is noted best in ECG lead V1. In contrast, the typical finding in patients with AV reentry caused by retrograde conduction over an accessory pathway (Wolff-Parkinson-White syndrome) is shown in Fig. 39–2. Note that the P wave is positioned in the early ST segment.
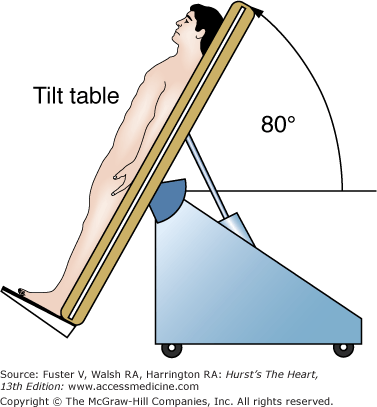
Head-up tilt (HUT) table testing is a diagnostic technique to assess the susceptibility of an individual to neurally mediated syncope.3 The protocol3 for HUT generally involves footrest-supported head-up tilting at 70 to 80 degrees for 30 to 45 minutes (Fig. 39–3). If HUT is negative, the test may be repeated following pharmacologic provocation. Several agents including sublingual nitroglycerin,4 epinephrine,5 and adenosine have been studied; however, most laboratories use isoproterenol at a dose of 1 to 3 μg/min. Repeat tilting is generally performed for 10 minutes after a steady state has been reached. Higher doses of isoproterenol, especially when coupled with longer durations of tilt, significantly decrease the specificity of the test.3
In control patients with no history of syncope, 70-degree head-up tilting has a specificity of approximately 90%.6 In patients with unexplained syncope, HUT can yield a diagnosis in 40% to 64%,7-10 particularly in the absence of other structural heart disease.
Risk stratification after MI may be divided into two categories. The first category includes signal-averaged ECG (SAECG) to identify high-frequency potentials at the end of the QRS complex (late potentials) and microvolt changes in T wave amplitude. The second category assesses autonomic tone by analyzing spontaneous and induced changes in heart rate and blood pressure. Although research continues with these tests, most currently have limited clinical utility.
SAECG allows the identification of small potentials in the surface ECG that are not seen because their amplitude is less than the noise intrinsic to the ECG signal.11 A more detailed description of the technique may be found elsewhere.12 In brief, orthogonal surface XYZ ECG leads are acquired for approximately 200 beats and digitally stored. High-pass filtering minimizes the contribution of low-frequency content. The X, Y, and Z leads are then combined into a vector magnitude referred to as the filtered QRS complex (Fig. 39–4). Most commonly, the SAECG has been used to identify late potentials appearing at the end of the QRS complex. These potentials correspond to fragmented electrical activity that is generated in areas of slow conduction either within or at the border zone of infarcts, which may be arrhythmogenic and prone to reentry.13
Figure 39–4
Positive signal-averaged electrocardiogram in a patient with sustained ventricular tachycardia. All three measured parameters are abnormal. Filtered QRS duration (DUR) is 136 ms, and the root-mean-square (RMS) voltage of the last 40 ms of the QS complex is 4.37 μV. LAS, low-amplitude signal. Reproduced with permission from Prystowsky EN, Klein GT. Cardiac Arrhythmias: An Integrated Approach for the Clinician. New York, NY: McGraw-Hill; 1994:345.
Three parameters have been identified to describe late potentials:
Filtered QRS duration (QRSd)
Root-mean-square voltage of the terminal 40 ms of the QRS complex (RMS40)
The duration of the low-amplitude signal (LAS) >40 mV
The latter two parameters represent the amplitude and duration of the late potential, respectively. With 40-Hz filtering, a QRSd >114 ms, RMS40 <20 mV, and LAS >38 ms are considered abnormal.11 The interpretation of the SAECG is problematic in the presence of a significant baseline intraventricular conduction defect.
Late potentials are hypothesized to be associated with an increased incidence of ventricular arrhythmias and sudden death.14-17 In some studies of patients after MI, the incidence of an arrhythmic event was 17% to 29% when late potentials were present. When late potentials were absent, the incidence of sudden death was 3.5% to 5%. Late potentials were an independent risk factor when assessed along with left ventricular ejection fraction (LVEF).16 The combination of an abnormal SAECG, reduced ejection fraction, and high-grade ectopy identified a population with a 50% risk in the same study. The identification of late potentials is one of the diagnostic criteria for arrhythmogenic right ventricular dysplasia (ARVD).18 A recent study showed that abnormalities in the SAECG correlated with extent of disease in patients with ARVD.19
Microvolt T wave alternans (MTWA) is a technique that measures small changes in T wave amplitude that occur on an alternating beat-to-beat basis. These changes have been associated with an increased risk of malignant ventricular arrhythmias20 and are thought to be caused by exaggerated repolarization heterogeneity.21 Several techniques22 have now been developed to detect and quantify these subtle variations in T wave amplitude. The most common method involves the spectral analysis of a large number of beats. Using this technique, an alternans power and alternans voltage can be determined. The alternans voltage represents the magnitude of the variation of the alternans T wave amplitude from the mean T wave amplitude.
Rosenbaum and colleagues23 reported the prospective assessment of MTWA in the prediction of sudden death and inducibility at electrophysiologic study in 83 patients. The presence of MTWA was an independent predictor of inducibility at the electrophysiology study (relative risk [RR] = 5.2). Of 66 patients followed for up to 20 months, 13 had an arrhythmic event. The 20-month arrhythmic-free survival was 19% when MTWA was present and 94% when MTWA was absent.
Because the prognostic value of MTWA appears to be heart rate dependent, current techniques are based on the development of MTWA during treadmill exercise.24,25 Patients who developed MTWA at lower heart rates had a higher risk. Patients who developed MTWA only at high heart rates or did not develop MTWA were felt to be at lower risk.26
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree