Aortic Valve Replacement
David A. Fullerton
THE SURGICAL ANATOMY OF THE AORTIC VALVE
The normal aortic valve is composed of three thin, pliable leaflets or cusps attached to the heart at the junction of the aorta and the left ventricle. The leaflets are attached within the three sinuses of Valsalva to the proximal aorta and joined together in three commissures that create the shape of a coronet. Because the coronary arteries arise from two of the three sinuses of Valsalva, the aortic leaflets are named after their respective sinuses as the left coronary leaflet, the right coronary leaflet, and the noncoronary leaflet. However, because of the oblique position of the aortic root, the sinuses themselves are rarely in a strict left or right position. The attachment of the leaflets to the left ventricular outflow tract is termed an annulus; however, in the strictest terminology this is not a true annulus because it is not truly circular: the points of attachment of the leaflets do not all lie in the same plane. There are two important surgical landmarks. First, the commissure between the left and noncoronary leaflets is positioned along the area of aortic–mitral valve continuity. Beneath this commissure is the fibrous subaortic curtain. The commissure between the noncoronary and the right coronary leaflets is positioned over the left Bundle of His. Injury to this conduction bundle during aortic valve surgery may create heart block.
INDICATIONS
Aortic Stenosis
The diagnosis and severity of aortic stenosis are determined by echocardiography. The normal aortic valve area (AVA) is approximately 3 to 4 cm2, and it has very little gradient across the valve until the AVA has been reduced by approximately one-half. Therefore, the flow velocity across the normal aortic valve (determined by Doppler echocardiography) is ≤1.0 m/s. With mild aortic stenosis, the AVA is decreased to >1.5 cm2, and the flow velocity across the valve is increased to 2.5 to 2.9 m/s. Aortic stenosis is considered moderate when AVA is reduced to 1.0 to 1.5 cm2, and the flow velocity across the valve increases to 3.0 to 4.0 m/s. Severe aortic stenosis is diagnosed by an AVA <1.0 cm2 and a velocity across the valve of >4.0 m/s. When normalized for patient body surface area (BSA), severe aortic stenosis is an AVA ≤0.60 cm2/m2.
Over a period of years, the valve progressively narrows. During this “latent” period, patients are typically asymptomatic. However, the progressive narrowing of the valve is not linear; it occurs in an unpredictable, stepwise manner. Patient survival is not significantly diminished until patients develop symptoms. Thereafter, survival is quite limited. The three principal symptoms of aortic stenosis are angina, syncope, and heart failure. With the onset of angina, the mean survival of a patient with aortic stenosis is 4.7 years. Once a patient develops syncope, the mean survival is typically <3 years. Patients with dyspnea and heart failure have a mean survival between 1 and 2 years. Heart failure is the presenting symptom in at least one-third of patients with aortic stenosis. Once a patient crosses the threshold from asymptomatic to symptomatic, approximately 3% to 5% of patients will die within weeks to months. Hence, it is extremely important to accurately identify the presence of symptoms. The presence of symptoms in a patient with aortic stenosis is an indication for an aortic valve replacement (AVR). Management of the asymptomatic patient is discussed below.
Aortic Regurgitation
The diagnosis and severity of aortic regurgitation are also determined by echocardiography. Patients with mild-to-moderate aortic regurgitation are typically asymptomatic and have an excellent prognosis without surgery. Severe aortic regurgitation may produce symptoms of heart failure, and AVR is indicated in the symptomatic patient. The asymptomatic patient is discussed below.
 SURGICAL TECHNIQUE
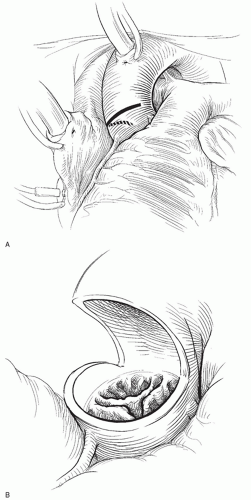
SURGICAL TECHNIQUEThe standard surgical approach for AVR is via a median sternotomy (Fig. 48.1). Cardiopulmonary bypass is established by aortic and right atrial cannulation. After initiation of cardiopulmonary bypass, the aortic root is vented and a left ventricular vent is inserted via the right superior pulmonary vein. If the aortic valve is competent, cardiac arrest may be achieved by antegrade cardioplegia with subsequent administration of cardioplegia in retrograde manner. Otherwise, all cardioplegia may be administered retrograde. The myocardial temperature is monitored in the interventricular septum and kept below 10°C by administration of cold blood cardioplegia every 20 minutes throughout the period of aortic occlusion. I routinely cool the patient to a bladder temperature of 28°C.
There are several important technical caveats. First, it is important to prevent the introduction of air into the left atrium with the insertion of left ventricular vent via the right superior pulmonary vein. This can be done by temporarily pinching the venous line, thereby filling the left atrium with blood. I typically vent the aortic root before placing the left ventricular vent to evacuate any air that might be introduced. Second, the ascending aorta in patients undergoing AVR may be very thin because of poststenotic dilatation, advanced age, annuloaortic ectasia, etc. Hence, aortic cannulation stitches must be placed very carefully to avoid tearing the aorta. I often use felt pledgets to reinforce each bite of the aortic cannulation stitches to minimize this problem. Third, when operating for aortic regurgitation, the heart is prone to ventricular fibrillation once cardiopulmonary bypass has been initiated. If the heart fibrillates, the left ventricle will
immediately distend, which may sometimes be lethal. To avoid this, I avoid systemic cooling until the left ventricular vent has been placed. The heart typically fibrillates soon after the initiation of systemic cooling, at which point I immediately cross-clamp the aorta and administer retrograde cardioplegia.
immediately distend, which may sometimes be lethal. To avoid this, I avoid systemic cooling until the left ventricular vent has been placed. The heart typically fibrillates soon after the initiation of systemic cooling, at which point I immediately cross-clamp the aorta and administer retrograde cardioplegia.
Once cardiopulmonary bypass has been initiated, the plane between the aorta and pulmonary artery is dissected. This is important to optimize visualization of the aortic valve and to facilitate aortic closure. One of the most important technical nuances of this operation is to identify the surface anatomy of the right coronary artery as it originates from the right sinus of Valsalva. This may be done by gentle dissection of the fat pad overlying the right sinus of Valsalva. One must be sure that the aortotomy is not too close to the right coronary ostium, for the ostium may be damaged or distorted with aortic closure or by the valve itself. Once the origin of the right coronary artery is identified, I mark the aorta with the electrocautery at the point of the anticipated aortotomy, approximately 2 to 2.5 cm distal to the origin of the right coronary artery.
As the patient is systemically cooled, the heart will fibrillate. The aortic cross-clamp is then applied, and cardioplegia is administered.
A small, transverse aortotomy is made at the point of the previously placed mark. Through this initial aortotomy, one may visualize the aortic valve. The aortotomy is then extended transversely across the anterior surface of the aorta. It is important to stay approximately 1 cm distal to the zenith of the commissures of the aortic valve leaflets. Having extended the aortotomy to the patient’s right, once the incision is exactly over the half-way point of the noncoronary leaflet, the aortotomy incision is directed to the axis of the aorta down toward the aortic annulus. This portion of the aortotomy incision should stop at least 1 cm distal to the aortic annulus.
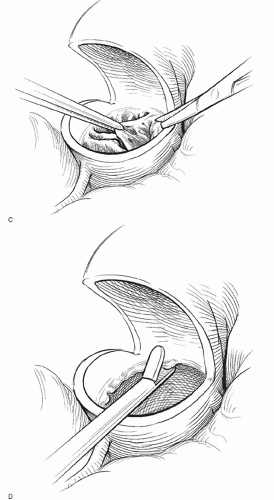
The aortic valve is best visualized with the operating table in a bit of reverse Trendelenberg position and rotated a bit to the patient’s left. Traction sutures are then placed through the top of each commissure and snapped to the surgical drapes. This brings the aortic valve up toward the surgeon. The aortic valve leaflets are then excised with scissors. After the leaflets have been removed, a moist gauze sponge is placed in the lumen of the left ventricle to help catch any small pieces of calcium. A Rongeur instrument is then used to gently debride the annulus of calcium.
During this process, it is helpful for the assistant to follow along with an opentipped suction catheter to help sweep up any small pieces of calcium. Once the annulus has been sufficiently debrided of calcium, the sponge is removed from within the ventricle, and the lumen of the left ventricle is liberally irrigated with cold saline to flush out any calcium debris. The annulus is then sized.
During this process, it is helpful for the assistant to follow along with an opentipped suction catheter to help sweep up any small pieces of calcium. Once the annulus has been sufficiently debrided of calcium, the sponge is removed from within the ventricle, and the lumen of the left ventricle is liberally irrigated with cold saline to flush out any calcium debris. The annulus is then sized.
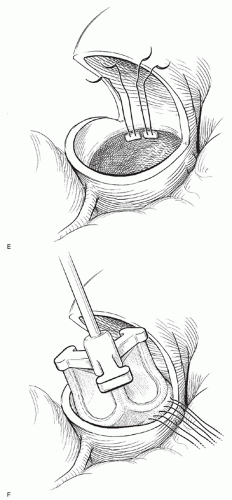
It is very important not to attempt to place an oversized valve. Regardless of the choice of prosthesis, I routinely implant a valve one size smaller than the patient’s annulus might accept as judged by valve sizers. Horizontal pledgetted mattress sutures are placed in the aortic annulus with the pledgets in the subannular position. Note that the annular size will be somewhat smaller once all the valve sutures have been placed. Therefore, I finalize the choice of valve size only after all the valve sutures have been placed. The aortic valve prosthesis is then brought to the field and the sutures passed through the valve sewing ring. To facilitate symmetrical suture placement, it is helpful to mark the sewing ring in thirds.
Once the sutures are passed through the sewing ring, the valve is seated into the aortic annulus, and the sutures tied. To minimize difficulty in seating the valve, the sutures at each of the three commissures should be tied first. Next, a suture midway between each commissure should be tied. In this manner, the surgeon may be assured that the valve will seat appropriately.
Once the valve is sewn in place, the aortotomy is closed with 5-0 polypropylene sutures in two layers. The first layer is a running horizontal mattress stitch, and the second is an over-and-over running stitch. Once the aortotomy is closed, I routinely administer antegrade cardioplegia in order to test the suture line. It is easier and safer to repair any leaks before removal of the cross-clamp.
In anticipation of removing the aortic cross-clamp, I typically infuse warm blood in retrograde manner. The purpose of this is to flush air out of the coronary arteries and to begin increasing the myocardial metabolic rate before reanimating the heart.
During this infusion, it is helpful to begin de-airing the left ventricle by partial occlusion of the venous line and the resumption of ventilation while the left ventricular and aortic vents are on suction. After the retrograde administration of 500 cm3 of warm blood, I administer warm blood in an antegrade manner until the heart reanimates. With the heart beating, the aortic cross-clamp is removed. With the heart beating, one should assess the adequacy of the de-airing maneuvers by transesophageal echo. The usual maneuvers include filling the heart while on cardiopulmonary bypass, rotating the operating table from right-to-left and Valsalva maneuvers to express air out of the pulmonary veins. Once satisfied that the left heart is completely de-aired, the
left ventricular vent is removed and the patient is weaned from cardiopulmonary bypass.
left ventricular vent is removed and the patient is weaned from cardiopulmonary bypass.
SURGICAL OUTCOMES
According to the Society of Thoracic Surgeons (STS) National Cardiac Surgery Database in 2010, approximately 35,000 valve operations are performed in the United States annually; just over one half are cases of isolated AVR. The operative mortality rate in the STS Database for isolated AVR is approximately 2.6%, and the incidence of stroke is 1.3%. The operative mortality rate for combined AVR/CABG is approximately 6.8%.
Certain patient-specific risk factors markedly increase the odds ratio (OR) of death following AVR. Operative mortality is significantly increased with surgery performed under salvage status conditions (OR 7.12), dialysis-dependent renal failure (OR 4.32), emergency status (OR 3.46), nondialysis-dependent renal failure, and first reoperation (OR 1.70).
CHOICE OF PROSTHETIC AORTIC VALVE
The operative risks of cardiac valve replacement are not associated with the choice of prosthesis. Further, the hemodynamic performances of contemporary valves are similar. Traditionally, the choice of aortic valve prosthesis has focused on whether a patient would be committed to the risks of life-long anticoagulation (mechanical valve) or the presumed need for reoperation for structural valve deterioration (bioprosthetic valve). However, this approach is an over simplification and the choice of prosthetic valve must be patient-specific. In addition to appropriate consideration of a given patient’s comorbidities, one of the most important considerations is the age of the patient at valve implantation. In this light, it is important to recognize that approximately 80% of all valve replacements in the United States are performed in patients above the age of 60 years. Currently, the use of bioprosthetic valves has risen to 80% of all implanted valves.
Following AVR, the median survival for patients <80 years is approximately 11 years; for octogenarians, it is approximately 6 years. The 10-year survival for patients following AVR ranges from 40% to 70%, with an average in the literature of 50%. The type of prosthesis does not impact survival, but other patient-specific factors such as age at operation and presence or absence of coronary artery disease do impact survival following valve replacement.
Regardless of the type of prosthetic valve implanted, approximately one-third of patients die of valve-related causes. Given that valve-related complications occur at a frequency of about 3% to 6% per year, it is important to ask if the risks in a specific patient may be minimized by the choice of a mechanical versus a bioprosthetic valve. The choice of valve prosthesis must be individualized.
Regardless of the type of prosthetic valve implanted, approximately one-third of patients die of valve-related causes. Given that valve-related complications occur at a frequency of about 3% to 6% per year, it is important to ask if the risks in a specific patient may be minimized by the choice of a mechanical versus a bioprosthetic valve. The choice of valve prosthesis must be individualized.
Mortality among heart valve recipients is valve-related in approximately 30% of patients. The principal causes of valverelated death following valve implantation include thromboembolism (12%), reoperation (10%), bleeding (4%), and prosthetic valve endocarditis (PVE) (3%). The risk of PVE is not different between mechanical or tissue valves. It is approximately 4% spread over the patient’s lifetime. However, if PVE does occur, it is associated with up to a 50% mortality rate.
The leading cause of valve-related death is thromboembolism. Largely because mechanical valves are thrombogenic, the risk of thromboembolism is greater with mechanical valves. At 10 years following AVR, the risk of thromboembolism is 20% for mechanical valves and 9% for bioprosthetic valves.
Because a mechanical valve obligates the patient to chronic anticoagulation therapy (warfarin sodium), the choice of a prosthetic valve must consider the risks of chronic anticoagulation. The risk of bleeding complications from chronic anticoagulation is between 1% and 2% per year. In fact, 4% of valve-related deaths result from bleeding. Mechanical valves should be avoided in patients with contraindications to anticoagulation because of occupation or because of coexistent medical conditions. Similarly, patients who are medically noncompliant or whose level of anticoagulation may not be closely monitored should not receive mechanical valves.
Ten percent of valve-related deaths result from reoperation. It was traditionally assumed that following implantation of a tissue valve, patients would require reoperation for structural valve deterioration. Mechanical valves were, therefore, recommended for patients with a life expectancy longer than 10 years. This reasoning requires refinement. First, placement of a mechanical valve does not eliminate the potential for subsequent valve reoperation. While mechanical valves will not structurally fail, approximately 10% of mechanical aortic valves require reoperation within 5 to 10 years, primarily for paravalvular leak, endocarditis, or nonstructural valve dysfunction such as scar tissue or pannus in-growth. Second, the structural durability of newer bioprosthetic valves is superior to prior generations of valves. Third, it is now appreciated that on average, patient death (all-cause mortality) occurs prior to reoperation for structural valve deterioration. In fact, the incidence of reoperation for structural valve deterioration of a bioprosthetic valve is <15% for patients older than 60 years.
THE ASYMPTOMATIC PATIENT
Asymptomatic Aortic Stenosis
In general terms, asymptomatic patients do not require AVR. However, some do carry a low but real risk of sudden death. The challenge is the identification of the subset of asymptomatic patients in whom the risk of death is greater if AVR is not performed, or in whom the likelihood of AVR in the nearterm is probable.
The Risks without Operation
The risk of sudden death in asymptomatic patients is considered to be quite low. However, in a rigorous study of nonoperated patients with asymptomatic aortic stenosis, the incidence of sudden death was approximately 6%. Further, one must also consider that the mortality is 3% to 4% soon after the onset of symptoms.
Unmasking the Symptoms
Given the patient with significant aortic stenosis by echocardiogram, it is therefore important to determine that the patient is truly asymptomatic. This may be objectively confirmed by exercise stress testing (treadmill). While unnecessary and risky in the symptomatic patient with aortic stenosis, exercise testing has been found safe in asymptomatic patients. A modified Bruce protocol should be employed under careful observation. An exercise test is considered positive if symptoms occur, systolic blood pressure falls by more than 10 mmHg, dysrhythmias occur, or ST segment changes are noted. Given a positive exercise test, the patient should be considered symptomatic and offered surgery. As many as 66% of asymptomatic patients may have a positive stress test.
Outcomes of Those with a Negative Stress Test
Following a negative stress, patients must be closely followed for hemodynamic progression of aortic stenosis. On average, the aortic flow velocity increases by 0.3 m/s per year and the AVA decreases by 0.1 cm2 per year. However, the flow velocity is largely dependent upon ventricular contractility and should ventricular function decline, velocity may not change despite a smaller AVA. Further, the rate of progression of aortic stenosis is quite variable, making it difficult to predict the clinical course of a given patient.
Several characteristics have been identified, which help stratify asymptomatic patients. Otto and colleagues identified aortic flow velocity as the most important patient-specific variable. The highest risk group were those asymptomatic patients with an aortic flow velocity of >4 m/s, as only 21% were alive without valve replacement at 2 years.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree