Aortic Valve Repair in Children
Chawki el-Zein
Anastasios Polimenakos
Michel N. Ilbawi
Surgical management of aortic valve disease in children presents a difficult dilemma. On the one hand, early surgery protects the myocardium from volume and pressure overload, decreases the chance of fibrosis and remodeling, and is consistent with current surgical philosophy of early complete repair of all congenital heart defects. On the other hand, early valve replacement in children is suboptimal because of the lack of an ideal valve substitute that allows growth and does not need anticoagulation or frequent replacement. Autologous pulmonary valve has emerged recently as an attractive aortic valve substitute that fulfills these criteria but concerns persist over the long-term fate of the pulmonary valve in the aortic position.
The dichotomy created by the absence of the ideal valve substitute and the deleterious effects of long-standing ventricular volume and/or pressure overload associated with aortic valve disease has renewed interest in aortic valvuloplasty especially in children. Although several techniques, such as annular reduction, commissural resuspension, and cusp extension were used in the past, aortic valvuloplasty remained an evolving approach rather than a definitive treatment, due in part to incomplete understanding of the functional anatomy and geometry of the aortic valve. Recently, success in atrioventricular valve repair, progress in myocardial protection, refinements in three-dimensional imaging of the aortic valve, and detailed analysis of valve anatomy and function have led to improved results of aortic valve reconstruction.
ANATOMY AND FUNCTION OF THE AORTIC VALVE
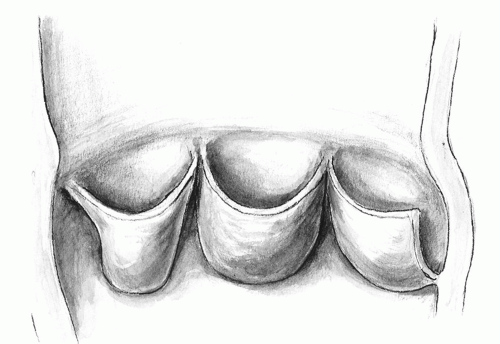
The three leaflets of the aortic valve are attached to the aortoventricular junction. The collagenous condensation at the point of attachment of each leaflet has been termed the annulus fibrosis. There is, however, no true “ring” of annular tissue supporting the leaflets in a straight circular plane. The hemodynamic stresses on the leaflets, therefore, are counteracted at several structural levels. The margin of coaptation of a competent valve is more than a finite point of contact. It extends along the whole margin of the leaflet in length and several millimeters in depth. Beneath the apices formed by leaflet attachment, the so-called commissures, there are subcommissural or interleaflet triangles (Fig. 103.1). The wide base of these triangles follows the ventricular contraction pattern and allows optimal retraction of leaflets during systole. The sinotubular bar marks the junction with the ascending aorta. It is thicker than the adjacent sinuses. It is circular with areas of increased collagen. It acts as a suspension post that supports the peripheral attachments (the commissures) of the valve leaflets. The parabolic shape of the leaflets resembles a suspension bridge. Their attachments to the sinotubular bar are several millimeters above the level of coaptation. As these support poles stretch outward by as much as 16% to 44% during early systole, the leaflet edges (the cables) become straighter, aiding in the opening of the valve.
The aortic root is also a complex hemodynamic system. Its component parts change in size and shape during the cardiac cycle. Its distal portion is exposed to the aortic pressure. It expands to allow leaflet retraction. Its base is exposed to ventricular dynamics. It contracts during the peak of systole to decrease the distance between the leaflets and to reduce the stress forces applied to leaflets in early diastole. Moreover, the leafletsinus assembly behaves as an independent unit to store the diastolic pressure within. It allows the aortic valve to remain competent even if the interleaflet triangles are partly incised. The instantaneous changes in aortic valve orifice have been shown to precede movement of blood in the ventricle. The transformation of the aortic orifice from a closed position to a triangle and then to a circle without causing flexion deformity of cusp tissue is related to aortic root distensibility and the mechanism of leaflet suspension.
PATHOLOGY AND FUNCTION OF THE ABNORMAL AORTIC VALVE
Aortic Valve Stenosis
The Congenital Bicuspid Aortic Valve
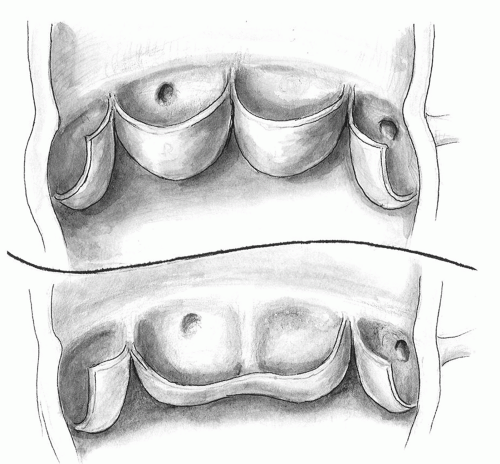
In type I, there is no median raphe at the junction of two cusps. As a result, there are two rather symmetric aortic sinuses and leaflet base attachment. The valve orifice is central. The commissural triangle is rather well developed. The leaflets are suspended at the sinotubular bar and have adequate depth. In type II, which is more prevalent, a median raphe is present. The cusps are asymmetric and the fused leaflet is longer, shallower, and takes up more of the circumference of the valve. In contrast to the normal tricuspid valve, the leaflet edges are excessive and sagging. As a result, there is increased folding and crossing and a compensatory extension of the area of leaflet approximation from their edges (doming). The opening of the valve is eccentric due to discrepancy in leaflet sizes. The orifice also has an elliptical rather than a circular opening. The resultant distortion in blood flow pattern exaggerates turbulence and predisposes to degenerative changes. Frequently, there is commissural fusion that limits the leaflet movement and further exaggerates the eccentricity of valve opening and the decrease in its effective orifice diameter. The narrowed opening, often combined with annular hypoplasia, impairs the ability of the leaflets to escape systolic or diastolic pressure load, further exaggerating the stress on the valve. The subcommissural triangle is severely attenuated. It limits leaflet movement in early systole and the change in orifice configuration necessary for appropriate leaflet coaptation at the end of systole. The leaflet edges are suspended below the sinotubular bar. This, combined with redundant leaflet edges, results in shallow sinuses, decreases coaptation area,
and exaggerates leaflet-deforming dynamic forces (Fig 103.2).
and exaggerates leaflet-deforming dynamic forces (Fig 103.2).
The Rheumatic Aortic Valve
The continued inflammatory process causes progressive scarring and thickening of the leaflets and fusion of the commissures. The valve becomes progressively stenotic.
Aortic Valve Regurgitation
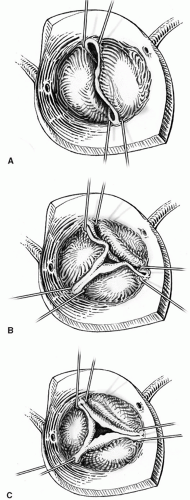
There are three types of regurgitant aortic valves. Type I is dilatation of the aortic annulus, sinotubular bar, or ventriculoaortic junction. Type II is leaflet prolapse. Type III is leaflet retraction and scarring. It is the most common pathology of the congenital regurgitant valve (Fig. 103.3).
Regurgitation Associated with Ventricular Septal Defect
There is discontinuity between the aortic media and the crest of the ventricular septum with consequent decrease in the support of the sinus wall and progressive prolapse and deformity of the involved cusp (Type II). The sagging leaflet edge loses coaptation contact with the other two leaflets and central regurgitation ensues. The noncoronary cusp is usually affected with perimembranous ventricular septal defects, whereas the right coronary cusp is involved with the subarterial, more anterior (supracristal) ventricular septal defect.
Regurgitation in Patients with Congenital Valvar Stenosis
The continued trauma to the leaflet edges produced by hemodynamic stress and abnormal flow patterns results in progressive scarring, thickening, deformity, and retraction of the leaflet edges and subsequent lack of coaptation (Type III).
Aortic Regurgitation Secondary to Subaortic Fibromuscular Stenosis
The abnormal blood flow pattern produced by the subaortic stenosis results in progressive deformity of the leaflet. Tethering of the leaflets by the subvalvar fibrous tissue, causing the obstruction, exaggerates the regurgitation (Type III).
Regurgitation in Marfan Syndrome
The pathology is progressive dilation of the aortic root wall due to fragmentation of its elastic support. The dilated sinotubular bar and valve sinuses stretch apart the commissural suspension and leaflet edges. The increase in hemodynamic stress due to changes in the leaflet suspension mechanism combined with enlarged aortoventricular junction leads to poor leaflet coaptation and central regurgitation (Type I).
Postballoon Regurgitation
This condition is usually caused by leaflet(s) tear close to the fused commissure. The leaflet becomes flail and eccentric regurgitation results (Types II and III).
Regurgitation after Arterial Switch Operation
Regurgitation in these cases is related to disruption of the sinotubular mechanism and undue dilation of the aortic sinuses caused by the implantation of large coronary artery buttons or preoperative pulmonary artery banding. Delayed closure of ventricular septal defect associated with D-transposition of the great arteries also predisposes to long-term neoaortic regurgitation following the switch operation (Type I).
Aortic Regurgitation Secondary to Rheumatic Disease
There is cusp retraction secondary to inflammation and scarring. The hemodynamic sequelae result in progressive annular dilation and worsening of the regurgitation (Types II and III).
TIMING OF SURGICAL INTERVENTION
To achieve optimal short- and long-term results, surgical intervention should be timed appropriately. The decision relies on achieving the goals of valve surgery, which include relief of symptoms, restoration of exercise capacity, improved quality of life, and, most importantly, protection of the myocardium from chronic pressure and/or volume overload. Most of the reported guidelines for timing of valve surgery are based on studies in the adult population and on the premise that valve replacement is the only therapeutic option. These studies use mortality rates as a follow-up endpoint but fail to analyze myocardial performance and reserve several years postoperatively. They utilize as their database several single-center observational studies and very few prospective, randomized trials.
The introduction of and refinement in valvuloplasty techniques have prompted critical evaluation of these older guidelines for the timing of surgical intervention on the diseased aortic valve. The availability of a surgical alternative that avoids valve replacement or anticoagulation has liberalized the older rigid criteria. Although large-scale, long-term data on repaired valves are not available, there is unquestionable evidence that valvuloplasty extends the functional longevity of the native aortic valve in children and may safely delay the need for replacement, thus justifying earlier surgical intervention. Waiting for symptoms to appear or for ejection fraction to decrease prolongs the duration of ventricular pressure and volume overload and may lead to irreversible ventricular dysfunction.
Timing of valvuloplasty involves several two- and three-dimensional echocardiographic and Doppler-derived indices. For isolated aortic valvar stenosis, pressure gradients of 40 to 50 torr associated with progressive left ventricular hypertrophy or impaired exercise tolerance are indications for intervention. Measurement of effective valve orifice and the extent of valve pathology are also helpful in deciding the timing of surgery. In aortic valvar regurgitation, a diameter ratio of regurgitant jet to annulus of ≥0.4 and the progressive increase in indexed end-diastolic left ventricular dimensions for two consecutive measurements, if they exceed a Z-score of 3, have been found to correlate with early ventricular dysfunction before onset of symptoms, and therefore constitute valid and rather objective indications for intervention. This is especially pertinent as isolated data have shown that patients with significant dilatation of the left ventricular cavity have an increased incidence of recurrent aortic insufficiency even with a successful valvoplasty. However, in specific situations such as balloon-induced aortic regurgitation, there are probable benefits for delaying surgery if the residual pathology is not severe. Remodeling of the leaflets and enlargement of the annulus from increased blood flow during the waiting period may improve the long-term outcome of the valvuloplasty. Other parameters, such as changes in ejection fraction have also been used to time interventions; however, these have not been found to correlate objectively with optimal surgical outcome.
Techniques of Surgical Valvuloplasty
Most of the surgical techniques used for aortic valve repair were devised many years ago or have been used for a long time. Recent improved understanding of the valve pathology and pathophysiology and refinements in cardiopulmonary bypass and myocardial protection in children have made their successful application possible. In addition, several principles have evolved that helped in improving outcome. These include the following: (1) detailed pre-and intraoperative analysis of pathology is essential. This is best achieved by two-and three-dimensional echocardiography. Objective preoperative assessment, however, is rather difficult. Pliability of each cusp is estimated by the apparent change in its area from systole to diastole. Adequacy of cusp tissue for coaptation is estimated by measuring the curvilinear height of the leaflet. Three-dimensional echocardiography helps in measuring leaflet-free edge, the depth of the sinus, and areas of tissue deficiency or prolapse. (2) More than one technique needs to be performed to achieve both competence and relief of obstruction. The different steps in the procedure should be tailored to address the specific pathology types. (3) Reconstructive steps should be preceded by relief of obstruction as completely as possible. All areas of leaflet fusion or stenotic lesions should be relieved first even if that reduces leaflet support. Subsequent reconstructive steps of such leaflets should aim at restoring the normal morphology, function, and support.
(4) Repair of only one leaflet or cusp is inadequate and leads to early failure. (5) Fresh autologous tissues such as pericardium or fascia lata cannot withstand dynamic stress when used for repair and tends to retract and scar with time; therefore, glutaraldehyde fixation is necessary. (6) “Overcorrection” in cases of aortic valve incompetence might be needed, but excessive correction may lead to crowding and distortion of the repaired valve if the root is normal or smaller than normal in diameter. (7) Centralizing blood flow through the valve decreases turbulence and extends the longevity of the repair; therefore, tricuspidization of the valve is advantageous when possible. (8) It is essential to incorporate in the procedure the necessary steps that address the interaction between aortic root dynamics and valve mechanics, namely, maintaining annular and commissural flexibility and movement in order to avoid accelerated stress-induced valve degeneration. Mobilization of the subcommissural triangle and avoiding subtotal excision of the leaflets close to the aortoventricular zone are important to avoid the disruption of the delicate and complex relationship between the root and leaflet. (9) Continued root dilatation whether at the aortoventricular or sinotubular bar junction induces recurrent aortic insufficiency and render the repair ineffective. The integrity of these junctions should be restored whenever they are dilated. (10) The abnormal ascending aorta should be replaced at the time of valvuloplasty especially in patients who demonstrate associated morphologic, histologic, or molecular abnormalities of the aortic wall.
(4) Repair of only one leaflet or cusp is inadequate and leads to early failure. (5) Fresh autologous tissues such as pericardium or fascia lata cannot withstand dynamic stress when used for repair and tends to retract and scar with time; therefore, glutaraldehyde fixation is necessary. (6) “Overcorrection” in cases of aortic valve incompetence might be needed, but excessive correction may lead to crowding and distortion of the repaired valve if the root is normal or smaller than normal in diameter. (7) Centralizing blood flow through the valve decreases turbulence and extends the longevity of the repair; therefore, tricuspidization of the valve is advantageous when possible. (8) It is essential to incorporate in the procedure the necessary steps that address the interaction between aortic root dynamics and valve mechanics, namely, maintaining annular and commissural flexibility and movement in order to avoid accelerated stress-induced valve degeneration. Mobilization of the subcommissural triangle and avoiding subtotal excision of the leaflets close to the aortoventricular zone are important to avoid the disruption of the delicate and complex relationship between the root and leaflet. (9) Continued root dilatation whether at the aortoventricular or sinotubular bar junction induces recurrent aortic insufficiency and render the repair ineffective. The integrity of these junctions should be restored whenever they are dilated. (10) The abnormal ascending aorta should be replaced at the time of valvuloplasty especially in patients who demonstrate associated morphologic, histologic, or molecular abnormalities of the aortic wall.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree