Introduction and historical perspective
Valvular heart disease affects more than 100 million patients worldwide and is a growing problem because of the increasing survival of patients with rheumatic heart disease in the developing world and the growing burden of degenerative valve disease in the aging population.1–2 Prosthetic valve replacement is the only definitive treatment for valvular heart disease and is performed in about 100 000 patients each year in North America.3 Thromboembolism is one of the most important complications of prosthetic heart valves and although the thrombogenicity of newer valves is less than with older valves, all patients undergoing valve replacement surgery require antithrombotic therapy.
This chapter reviews the evidence for antithrombotic therapy in patients with prosthetic heart valves and makes treatment recommendations based on the strength and quality of the evidence. The class of evidence reflects opinion about the usefulness of the treatment, and balances the positive effects of treatment against the negative effects on patient-important outcomes. Class I recommendations are strong and indicate that the benefits of the treatment outweigh the risks. Class II recommendations are weak and suggest that there are differences between patients in the balance of the benefits and risks of treatment. Class III recommendations indicate that the treatment does not have any benefits or that the risks outweigh the benefits. The level of evidence reflects the quality of the evidence. Level A is high-quality evidence from well-conducted randomized controlled trials or meta-analyses of well-conducted randomized trials with clear results. Level B is evidence from randomized trials or overviews of randomized trials that have significant limitations. Level C1 is evidence from high-quality and persuasive cohort studies, case–control studies or case series while Level C2 is lower quality evidence from non-randomized studies or opinions of experts.
Mechanical valves
Mechanical heart valves comprise three major components: an occluder (closure mechanism), housing and sewing ring.4 There are three main types of mechanical valves: caged ball, single leaflet/tilting disk and bileaflet valves (Figure 60.1). All mechanical valves have some degree of regurgitant flow that acts as a “washing jet” to prevent thrombus formation on the surfaces of the valve.
Figure 60.1 Different models of mechanical valves. (a) Starr–Edwards caged ball (courtesy of Edwards Lifesciences LLC, Irvine, California). (b) Bjork–Shiley tilting disk (courtesy of Sorin Group of Canada Inc.). (c) Medtronic Hall tilting disk (© Medtronic Inc. Printed with permission.). (d) St Jude Medical bileaflet (courtesy of Sorin Group of Canada Inc.).
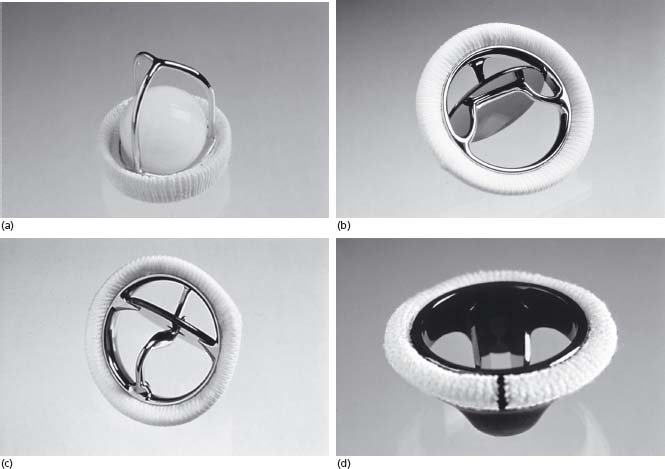
Caged-ball valves
The first prosthetic heart valve was the Starr–Edwards caged-ball valve introduced in 1960.5 The original version of the Starr–Edwards valve had a silicone rubber (Silastic) ball or poppet that freely moved within the confines of a three-strut alloy cage (Fig. 60.1a). Subsequent models had a metal ball and a four-strut cage. The free ball design theoretically prevents thrombus growth from the sewing ring onto the occluder6 but the ball generates a large wake of stagnant flow that may account for the high risk of thromboembolism associated with caged-ball valves.7 The Starr–Edwards valve was the “gold standard” against which new mechanical valves were compared for more than 20 years.8
Single leaflet/tilting disk valves
Single leaflet/tilting disk valves consist of a major orifice and a minor orifice. The single tilting disk enables central flow of blood, which may reduce the risk of thromboembolism compared to the circumferential blood flow of caged-ball valves. Single tilting disk valves appear to be associated with a slightly higher risk of thromboembolism than bileaflet mechanical valves, possibly because they have a region of stagnant blood flow located adjacent to the wall immediately downstream from the minor orifice and because the regurgitant washing jet volume is lower than for bileaflet valves.7
The first successful tilting disk valve was the Bjork–Shiley valve introduced in 1969.9 This valve consists of a single leaflet of pyrolytic carbon held in place by large inflow and small outflow alloy struts encircled by a Teflon sewing ring6 (Fig. 60.1b). The Bjork–Shiley convexoconcave valve was withdrawn in 1986 because of several cases of strut fracture and embolization of the disk.6 Figure 60.1c displays the Medtronic Hall single leaflet/tilting disk valve. Table 60.1 lists other models of single leaflet/tilting disk valves.
Table 60.1 Models of prosthetic valves
| Type of valve Mechanical | Model name |
Mechanical | |
Caged ball | Starr–Edwards |
| Magovern-Cromie | |
| Smeloff–Cutter | |
Single leaflet/tilting disk | Bjork–Shiley |
| Medtronic–Hall | |
| Omniscience, Omnicarbon | |
| Sorin Allcarbon | |
| Beall | |
| Cooley–Cutter | |
| Cross–Jones | |
| Gott Daggett | |
| Harken | |
| Kay–Shiley | |
| Starr–Edwards Disc | |
| AorTech Ultracor | |
| Wada–Cutter | |
Bileaflet | St. Jude Medical |
| ATS Open Pivot | |
| Carbomedics Standard, TopHat, Orbis, Optiform | |
| Edwards Duromedics, Tekna, Mira | |
| Medtronic Parallel | |
| MCRI On-X, Conform-X | |
| Sorin Bicarbon | |
Bioprosthetic | |
Porcine | Medtronic Hancock Standard, Hancock II, Hancock |
| MO, MO II, MI, Mosaic, Ultra | |
| Carpentier–Edwards, SAV | |
| St. Jude Medical Biocor | |
| Tissuemed Angell–Shiley | |
Pericardial | Carpentier–Edwards Perimount |
| Sorin Mitroflow, Pericarbon | |
| Ionescu–Shiley Standard, Low Profile | |
| Hancock | |
| Labcor–Santiago | |
Stentless | CryoLife stentless porcine |
| Edwards Prima Plus stentless porcine | |
| Medtronic Freestyle Aortic Root porcine | |
| St. Jude Medical Toronto stentless porcine | |
| St. Jude Medical Quattro Valve |
Bileaflet valves
The St Jude Medical valve introduced in 197710 was the first bileaflet valve and is the single most commonly implanted mechanical valve. Made of pyrolytic carbon coated with graphite, it consists of two leaflets hinged on a ring (Fig 60.1d). Encircling this structure is a sewing ring. The bileaflet valves listed in Table 60.1 have a similar design. Bileaflet valves provide symmetric, relatively non-turbulent, central blood flow.6
Bioprosthetic valves
Most bioprosthetic valves are porcine or made from bovine pericardium that is fashioned into valve leaflets. The valves are preserved in glutaraldehyde and mounted on a frame or stent made of metal or plastic covered with fabric that acts as the sewing ring.11 Bioprosthetic valves mimic native heart valves more closely than mechanical valves because they have unobstructed central flow which provides excellent hemodynamics. The surfaces of bioprosthetic valves also are less thrombogenic than mechanical valves and do not require lifelong anticoagulant therapy. Porcine and pericardial (bovine) valves do not differ importantly in thrombogenicity.
Porcine bioprosthetic valves
Porcine valves are the most widely used bioprosthetic valves.8 The first commercial porcine valve was the Hancock valve introduced in 1970. Figure 60.2 a displays the Medtronic valve and Figure 60.2b displays a freestyle model consisting of a porcine valve housed within its native aorta. Table 60.1 lists other types of porcine valves.
Figure 60.2 Different models of bioprosthetic valves. (a) Medtronic HK II Ultra porcine. (b) Medtronic Freestyle porcine (© Medtronic Inc. Printed with permission.). (c) Carpentier–Edwards Perimount bovine pericardial (courtesy of Edwards Lifesciences LLC, Irvine, California).

Bovine pericardial bioprosthetic valves
Bovine pericardial valves have several theoretic advantages over porcine valves. The valve leaflets are larger, which accommodates shrinkage over the life of the valve; leaflet opening is more complete and symmetric, which improves valve hemodynamics; and the collagen content of the valves is higher, which improves valve durability. The Carpentier–Edwards Perimount valve (Fig. 60.2c) is the only pericardial valve presently available in North America. It is unclear whether the theoretic advantages of pericardial over porcine valves translate into improved outcomes for patients. Bovine valves appear to be at least as durable as contemporary porcine valves.12,13
Oral anticoagulants
Vitamin K antagonists (VKAs) are the only oral anticoagulants available for clinical use and warfarin is the most widely used VKA in North America. Other VKAs commonly used in Europe include acenocoumarol and phenprocoumon. VKAs are challenging to use in clinical practice because they have a slow onset and offset, narrow therapeutic window, and variable dose response and are subject to numerous food and drug interactions.14 Thus close laboratory monitoring of the anticoagulant effect of VKAs is required. The international normalized ratio (INR) is a standardized method of reporting the intensity of anticoagulant therapy with VKAs adopted in 1982.15,16 Studies completed prior to 1982 often reported anticoagulant intensity based on the ratio of the patient’ s plasma value to that of plasma from a healthy control and it is difficult to compare these results with subsequent studies that reported anticoagulant intensity based on the INR.
Starting dose of VKA for patients requiring oral anticoagulant therapy
One randomized trial has studied the optimal starting dose of VKA for patients who have undergone valve replacement. Ageno et al randomized, in an open-label fashion, 197 patients after heart valve replacement to an initial 2.5mg (n = 84) versus 5mg dose of warfarin (n = 113).17 The INR was measured daily and the dose of warfarin was adjusted according to the result of the INR in the 5 mg starting dose group. The dose of warfarin was not modified in the 2.5 mg group until day 3 and then only if the INR was less than 1.5 or greater than 3.0. The primary outcome of the study was the proportion of patients with an INR of >2.6 within 6 days of surgery. A greater percentage of patients in the 5 mg than the 2.5 mg group had an INR level >2.6 within 6 days of surgery (42% v 26%, P < 0.05). There was no difference in the rate of bleeding between the two groups although the study was not powered for clinical outcomes (Level A).
Incidence, natural history and prognosis
Thromboembolism after mechanical valve replacement without antithrombotic therapy
Information concerning the risk of thromboembolism after mechanical valve replacement in patients not treated with anticoagulants primarily comes from small case series of patients who had a contraindication to VKAs. Cannegieter and colleagues performed a systematic review in which they examined the risk of thromboembolism and bleeding in patients with mechanical heart valves.18 Included in the review were seven studies involving 460 patients who did not receive oral anticoagulant therapy. During 1225 patient-years of follow-up the rates of throm-boembolism per 100 patient-years were (95% confidence intervals (CI) in parentheses): valve thrombosis 1.8 (0.9–3.0), major embolism 4.0 (2.9–5.2), total embolism 8.6 (7.0–10.4). The risk thromboembolism was reduced by more than 75% in a parallel group of patients who received VKA therapy (Level C1).
Thromboembolism after bioprosthetic valve replacement
The largest study of thromboembolism risk after bioprosthetic valve replacement is by Heras and colleagues who followed 816 patients with aortic, mitral or combined aortic and mitral bioprosthetic valves for a median of 8.6 years.19 The rate of thromboembolism during the first 10 days among patients who did not receive any antithrombotic therapy was approximately 50% for aortic valves and 60% for mitral valves. Patients who were commenced immediately postoperatively on VKA therapy had higher rates of thromboembolism during the first 10 days (aortic 41%, mitral 55%) compared with between day 11 and 30 (aortic 3.6%, mitral 10%) and after day 30 (aortic 1.9% per year, mitral 2.4% per year) (Level C2). The unexpectedly high rate of early thromboembolism in the study by Heras and colleagues is unexplained and is inconsistent with other reports. Multiple small cases series of patients with bioprosthetic valves have reported thromboembolism rates of between 0.7% and 2.8% per patient-year during long-term follow-up ranging from 18 months to 6 years.20–21,22,23,24,25,26 Most patients in the latter studies received oral anticoagulants, antiplatelet therapy or a combination of anticoagulants and antiplatelet therapy for 2–3 months after surgery (Level C2).
Factors that contribute to the thrombogenicity of prosthetic heart valves include altered blood flow and hemostatic activation caused by endothelial injury during surgery or exposure of artificial surfaces (sutures, sewing ring, occluder and valve housing) to the circulating blood.27 Because almost all prosthetic valves are stented, they have a smaller effective orifice area than the native valve that results in a transvalvular flow gradient. Stagnant flow may also result from growth of endocardial tissue (pannus) into the leaflets or valve mechanism. Endothelialization of foreign surfaces occurs over a period of about 3 months after which the risk of thrombosis decreases.21
In this section, we review the evidence for the benefits and risks of antithrombotic therapy in patients with prosthetic heart valves and make recommendations based on the usefulness of the treatment and the quality of the evidence. We focus on the results of high-quality studies (randomized controlled trials and meta-analyses of randomized controlled trials) whenever available and consider lower quality studies only when there are no high-quality studies.
Mechanical valves
Acute
Possible approaches to antithrombotic therapy immediately after mechanical heart valve replacement include aspirin, oral VKA therapy with or without initial “bridging” unfractionated heparin (UFH) or low molecular weight heparin (LMWH) therapy, or aspirin in combination with oral VKA therapy.
Oral VKA therapy
There are no randomized trials comparing oral anticoagulation to placebo (or no anticoagulation) in the immediate postoperative period. A systematic review by Kulik et al of primarily observational studies comparing different initial anticoagulation strategies involving 3056 patients who received VKA therapy immediately after surgery28 revealed an absolute rate of thromboembolism of 0.9% and a bleeding rate of 3.3% during the first 30 days (Level C1).
Oral VKA + LMWH
There are no randomized trials comparing VKA plus LMWH to VKA alone in patients with a mechanical heart valve. A prospective observational “before-and-after” study by Talwar and colleagues compared initial LMWH plus oral VKA therapy to VKA therapy alone in 538 patients.29
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


