Introduction
Acute coronary syndromes (ACS) are caused by rupture or erosion of an atherosclerotic plaque leading to exposure of plaque contents and constituents of the vessel wall to flowing blood.1 Driven primarily by the aggregation and adhesion of platelets to collagen and von Willebrand factor (vWF), subsequent thrombin generation promotes thrombus formation that may ultimately result in total or partial vessel occlusion. The principal aim of initial management in patients presenting with ST segment elevation myocar-dial infarction (STEMI) is to restore normal flow in the infarct-related artery as soon as possible.2,3 This has been shown to preserve left ventricular function4 and improve survival rate.5 Reperfusion may be achieved mechanically using percutaneous coronary intervention (PCI) or pharmacologically with a fibrinolytic agent. Given that thrombus formation and dissolution is a dynamic process, adjunctive antithrombotic therapy is important in both establishing and maintaining patency of the infarct-related vessel. In addition, antithrombotic agents help prevent complications of STEMI including deep vein thrombosis, pulmonary embolism and left ventricular thrombus. For the increasing number of patients treated with primary PCI, iatrogenic vessel injury and stent insertion can promote further local thrombosis and may result in acute vessel closure.6,7 The beneficial role of antithrombotic therapies, particularly antiplatelet agents, in reducing these events is well established.8–11 For those treated with a fibrinolytic agent, antithrombotic therapies are also important. Whilst fibrin-specific agents promote local clot lysis in the infarct-related vessel, they may result in a systemic prothrombotic state through enhanced thrombin generation12 and platelet activation.13
Although the development of antithrombotic agents has focused primarily on reducing thrombotic and ischemic complications, the importance of safety as well as efficacy of novel agents and regimens is increasingly recognized. Antithrombotic therapy is associated with an increased risk of bleeding and accompanying adverse outcomes, including death. The relationship between antithrombotic therapy, bleeding and ischemic events is complex. Whilst the adverse effects of fatal and intracranial hemorrhage may be clear, bleeding may also result in the premature cessation of antithrombotic therapy or necessitate blood transfusion which are themselves associated with an increased incidence of ischemic complications.
This chapter will examine evidence for antithrombotic use in patients with acute STEMI. Where possible, in order to enhance clinical relevance, discussion will be presented according to reperfusion strategy: those who have undergone primary PCI; those who have received fibrinolytic therapy; and those who have received no specific reperfusion therapy.
The role of platelets in STEMI
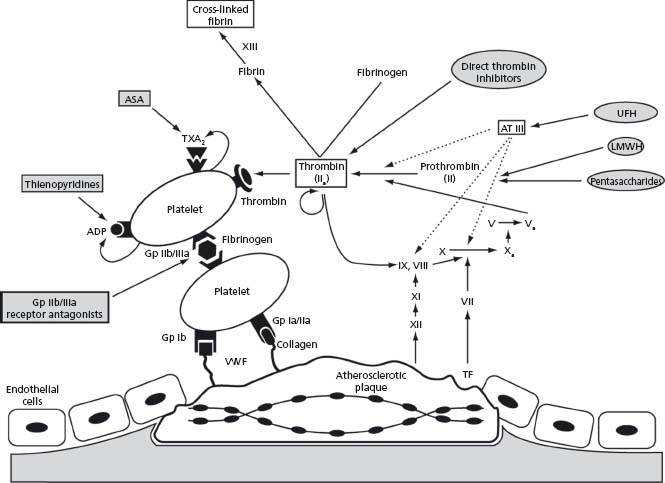
Platelets are central to the pathophysiology of arterial thrombosis (Fig. 32.1). Following rupture or erosion of an atherosclerotic plaque, platelet adhesion, activation and aggregation occur sequentially, culminating in formation of a platelet-rich thrombus. Platelet adhesion is mediated by the interaction of platelet surface receptors with exposed subendothelial proteins including vWF and collagen. Subsequent activation involves a conformational change in platelet shape from smooth discoid to spiculated, vastly increasing the surface area available for thrombin generation. Following conformational change, degranulation and secretion of prothrombotic and inflammatory mediators propagate and amplify the thrombotic process. The final step, platelet aggregation, involves glycoprotein IIb/IIIa receptor-mediated cross-linking of platelets via fibrinogen bridges.
Figure 32.1 Schematic diagram showing the mechanisms responsible for thrombus formation at the site of a ruptured atherosclerotic plaque and the site of action of antithrombotic agents. ADP, adenosine diphosphate; ASA, acetylsalicyclic acid; AT III, antithrombin III; G P, glycoprotein receptor; LMWH, low molecular weight heparins; TF, tissue factor; TxA2, thromboxane A 2; UFH, unfractionated heparin; VWF, von Willebrand factor.
Aspirin
Aspirin (acetylsalicylic acid, ASA) was the first antiplatelet therapy to be described and remains the principal agent used in the secondary prevention of all forms of cardiovascular disease. It exerts its antiplatelet effect by irreversibly inhibiting platelet cyclo-oxygenase 1. This results in decreased production of the platelet agonist and potent local vasoconstrictor, thromboxane A2.
The benefit of ASA in the context of acute myocardial infarction (MI) was established in the Second International Study of Infarct Survival (ISIS-2).14 This 2 × 2 double-blind trial randomized patients (n = 17187) to ASA (162.5mg daily for one month), streptokinase (SK, 1.5 million IU over one hour), both or neither. Aspirin reduced cardiovascular mortality at one month by 23% compared with placebo (9.4% versus 11.8%, relative risk reduction (RR) 23%, P < 0.00001). The benefit was maintained over at least 10 years of follow-up.15 Aspirin also reduced rates of reinfarction (odds ratio (OR) 0.51) and stroke (OR 0.49) compared with placebo without a significant increase in major bleeding or stroke.
A 1992 meta-analysis suggested that ASA (loading dose 162–325mg, maintenance dose 75–162mg/day) reduces the incidence of vascular events (cardiovascular death, MI and stroke) following STEMI by almost a third (relative RR 30%).16 Recent studies support earlier observations that compared to larger doses given long term, those in the range of 75–162 mg/day deliver efficacious antiplatelet activity17 but with improved side-effect and safety profiles.18
Aspirin should be administered immediately to all patients with STEMI at a dose of 162–325 mg and continued indefinitely at a maintenance dose of 75–162 mg/day. The only exception is true aspirin allergy (Class I, Level A).
Thienopyridines
Thienopyridines exert their antiplatelet effect by irreversibly inhibiting the binding of adenosine diphosphate (ADP) to the platelet P2Y12 receptor. As their mechanism of action is distinct from that of ASA, the effect of dual therapy is additive. Clopidogrel is currently the most widely used thienopyridine, being as efficacious as ticlopidine but possessing a more benign safety profile.19 Trials in patients with unstable angina (UA), non-STEMI18 and more latterly STEMI have established it as a key component of contemporary integrated management protocols for all patients presenting with ACS. Despite clear benefits, it has a number of important limitations including a delayed onset of action,20 a prolonged effect following cessation of therapy and inter-individual variation in antiplatelet effect21,22 that has been associated with an increased risk of ischemic events in poor responders.21,23 These limitations have prompted the development of novel agents, including prasugrel, capable of more rapid, consistent and efficacious inhibition of ADP-induced platelet aggregation than clopidogrel.
Thienopyridines in patients receiving fibrinolytic therapy
The use of clopidogrel in combination with a fibrinolytic agent was investigated in the CLARITY-TIMI-28 (Clopidogrel as Adjunctive Reperfusion Therapy (Thrombolysis In Myocardial Infarction)) trial.24 This study randomized patients (n = 3491) with STEMI to either clopidogrel (300 mg loading dose, followed by 75 mg/day) or placebo in addition to ASA and a standard thrombolytic regime. The initial clopidogrel dose was administered concurrently or within 10 minutes of fibrinolysis. All patients had coronary angiography performed prior to discharge. The primary endpoint (a composite of an occluded infarct-related artery at angiography or death/MI prior to angiography) occurred in 15.0% of those in the clopidogrel group compared with 21.7% of those receiving placebo (relative RR 31%, P < 0.001). At 30 days, clopidogrel reduced the odds of cardiovascular death, reinfarction or need for urgent revas-cularization by 20% compared with placebo (14.1% versus 11.6%, P = 0.03). The overall reduction in clinical events was similar to that in patients with UA and NSTEMI treated with clopidogrel.25 Encouragingly, there was no increase in TIMI major or minor bleeding. A subsequent analysis of patients who underwent coronary artery bypass grafting during the index admission found no increase in bleeding from randomization to the end of follow-up or from the time of surgery to the end of follow-up, including patients in whom clopidogrel was continued until within five days of surgery.26
The COMMIT/CCS-2 (Clopidogrel and Metoprolol in Myocardial Infarction Trial/Second Chinese Cardiac) Study also investigated the role of clopidogrel in a substantial cohort of patients with STEMI managed in non-interventional settings (n = 45852).27 Patients were randomized to clopidogrel 75 mg/day or placebo for up to four weeks (mean 15 days) in addition to ASA. In contrast to the CLARITY-TIMI-28 trial, no loading dose of clopidogrel was administered, patients > 75 years old were not excluded and the delay between symptom onset and fibrinolysis was longer (just under three hours in CLARITY-TIMI-28 compared with over 10 hours in COMMIT/CCS-2). In addition, only 55% received fibrinolysis (all with urokinase, a non-fibrin specific agent), whereas in CLARITY-TIMI-28 all patients received fibrinolysis (approximately two-thirds with a fibrin-specific agent and the remainder with SK). Despite the absence of a loading dose in COMMIT/CCS-2, there was a lower incidence of death, reinfarction or stroke during the index admission (9.3% versus 10.1% in the placebo group, relative RR 9%, P < 0.002). Rates of major bleeding were low and similar in those treated with clopidogrel and placebo (0.58% versus 0.55%, P = 0.59).
Despite differences in the design of the two studies, the results of CLARITY-TIMI-28 and COMMIT-CCS-2 demonstrate that addition of clopidogrel to standard therapy including ASA and fibrinolysis is safe and reduces the incidence of major vascular events and mortality.
Clopidogrel 75 mg/day should be administered to all patients with STEMI undergoing fibrinolytic therapy (Class I, Level A) and continued for at least 14 days (Class I, Level B).
For patients less than 75 years of age, it is reasonable to administer a loading dose of clopidogrel 300 mg (Class IIa, Level B).
Thienopyridines in patients undergoing primary PCI
Three trials, CREDO (Clopidogrel for the Reduction of Events During Observation),20 the PCI arm of the CURE (Clopidogrel in Unstable Angina to Prevent Recurrent Events) trial (PCI-CURE)25 and PCI-CLARITY, a substudy of the CLARITY-TIMI-28 trial, have considerably strengthened the evidence for early administration of clopidogrel alongside ASA in patients undergoing PCI in a wide range of clinical settings.
The PCI-CLARITY (Clopidogrel as Adjunctive Reperfusion Therapy) trial was a prospective subgroup analysis of patients (n = 1863) from the CLARITY-TIMI-28 study who underwent PCI following mandated angiography28 (PCI was performed between two and eight days following admission and not as the primary reperfusion strategy). As discussed above, a 300 mg loading dose of clopidogrel or placebo was administered at the time of fibrinolysis, in addition to standard therapy (ASA, fibrinolytic and unfractionated heparin (UFH) as appropriate). In contrast to other clopidogrel trials, patients in the control group also received clopidogrel (loading dose of 300–600 mg) after the diagnostic catheterization. All patients were given clopidogrel 75 mg/day for 30 days after the procedure. Thus, the trial compared pretreatment with clopidogrel with delayed administration at the time of PCI. In the pretreatment group, there was a 46% reduction in the odds of death, MI or stroke at 30 days compared with the delayed treatment group (3.6% versus 6.2%, P = 0.008). In addition, a 38% reduction in MI or stoke was observed prior to PCI (P = 0.028). The authors also performed a meta-analysis including data from CREDO (patients undergoing elective PCI) and PCI-CURE (patients with non-ST elevation acute coronary syndrome undergoing PCI) demonstrating a significant and consistent benefit of dual antiplatelet therapy initiated as early as possible prior to PCI.
Despite the size and consistency of data supporting the use of clopidogrel in the context of PCI, there have been no published studies specifically investigating its use in patients with STEMI undergoing primary PCI.
Limited data pertaining to the use of prasugrel in patients with STEMI undergoing PCI are available from the Trial to Assess Improvement in Therapeutic Outcomes by Optimizing Platelet Inhibition with Prasugrel–Thrombolysis in Myocardial Infarction (TRITON–TIMI) 38.29 Moderate- to high-risk patients (n = 13 608) with ACS (with and without ST segment elevation) who were scheduled for PCI were randomized to either clopidogrel (300 mg loading dose followed by 75 mg daily) or prasugrel (60 mg loading dose followed by 10 mg daily) in addition to ASA and followed for a mean of 14.5 months. A quarter of the study population had STEMI (n = 3534) although the proportion of this subgroup that underwent primary compared to delayed PCI was not specified. In the overall study population, prasugrel reduced cardiovascular death, MI and stroke (9.9% with prasugrel versus 12.1% with clopidogrel, P < 0.001) and resulted in a marked reduction in stent thrombosis (2.4% versus 1.1% with clopidogrel, P < 0.001). Whilst all-cause mortality, MI, stroke and TIMI major bleeding (taken as a marker of overall clinical benefit) were reduced in the prasugrel group (12.2% versus 13.9% with clopidogrel, P < 0.004) there was an excess of TIMI major bleeding (2.4% versus 1.8%, P = 0.03) and fatal bleeding (0.4% versus 0.1%, P = 0.002). The balance of risk appeared to be in favor of prasugrel during the first few hours and days when it reduced ischemic events with little adverse effect on bleeding. Following this initial period, however, its ability to reduce ischemic events was limited and associated in the longer term with an excess of major bleeding compared to clopidogrel. It should be noted that study drugs were administered following angiography, and therefore prasugrel was effectively being compared with placebo for the first few hours following randomization as conversion of clopidogrel to its active metabolite takes a number of hours.
Whilst the results from TRITON-TIMI-38 suggest that greater inhibition of platelet function can reduce ischemic events, there is an associated increase in major bleeding. Although a limited subgroup analysis has been performed to assess bleeding risk, further analysis of the existing data as well as forthcoming trials will be necessary to define more precisely the patient subgroups in whom most benefit is obtained as well as the optimal timing, duration and dose in patients with ACS including patients with STEMI.
Glycoprotein IIb/III a receptor antagonists
The glycoprotein (Gp) IIb/IIIa receptor antagonists inhibit the Gp IIb/IIIa integrin, found exclusively on platelets and megakaryocytes. The interaction of the receptor with its primary ligand, fibrinogen, is considered the final common pathway of platelet aggregation and blockade results in inhibition of aggregation irrespective of the initial agonist. There are close to 100 agonists known to stimulate platelet aggregation. Given the central role of platelets in arterial thrombosis and the fact that ASA and clopidogrel inhibit only the thromboxane A2 and ADP pathways respectively, the Gp IIb/IIIa receptor antagonists represent an attractive potential therapy in patients with STEMI.
There are three Gp IIb/IIIa receptor antagonists currently licensed for use. Abciximab is a chimeric (murine-human) monoclonal antibody directed against the IIb/IIIa receptor whereas tirofiban and eptifibatide are both small-molecule non-competitive IIb/IIIa inhibitors.
Gp IIb/IIIa receptor antagonists in patients receiving fibrinolytic therapy
The combination of a Gp IIb/IIIa receptor antagonist with a fibrinolytic agent (in full and reduced dose) has been evaluated extensively in efforts to improve the outcome from pharmacologic reperfusion.
Gp IIb/IIIa receptor antagonists and full-dose fibrinolytics
The relatively small Combined Accelerated t-PA and Platelet Glycoprotein Integrin Receptor Blockade with Integrillin in Acute Myocardial Infarction (IMPACT-AMI) study randomized patients (n = 180) to alteplase and either placebo or one of six doses of eptifibatide (in addition to ASA and UFH).30 The highest dose of eptifibatide (180μg/kg bolus followed by an infusion at a rate of 0.75 μg/kg/min) achieved higher rates of TIMI 3 flow at 90 minutes compared with placebo (66% versus 39%, P = 0.007). The accompanying major bleeding rates were 3.9% and 5.4% respectively. A further pilot study (n = 181) investigated the efficacy of three doses of eptifibatide (180μg/kg bolus followed by an infusion at a rate of 0.75, 1.33 or 2 μg/kg/ min) compared with placebo in combination with full-dose SK.31 There appeared to be an increase in the rate of TIMI 3 flow at 90 minutes with eptifibatide (44% for the combined eptifibatide groups versus 31% in the placebo group, P = 0.07). However, rates of bleeding were higher in those treated with the highest dose of eptifibatide compared with the two lower doses and placebo (17% versus 11% versus 0% respectively, P = 0.007).
In summary, these small angiographic pilot studies suggested that whilst the addition of Gp IIb/IIIa receptor antagonists to full-dose fibrinolytics was effective at increasing target vessel patency, there appeared to be an excess in bleeding.
Gp IIb/IIIa receptor antagonists and reduced-dose fibrinolytics
Angiographic studies
The TIMI-14 trial investigated abciximab use in combination with SK or alteplase.32 Patients (n = 888) were randomized to one of four treatment groups: alteplase only (100 mg); abciximab only (0.25 mg/kg bolus followed by a 12 h infusion of 0.125 μg/kg/min); abciximab with 20–65mg of alteplase; or abciximab with 0.5–1.5 million IU of SK. The highest rate of TIMI-3 flow was seen with abciximab in combination with 50 mg of alteplase (77% at 90 minutes versus 62% in the alteplase-only group, P = 0.02). Overall, there was no significant difference in major bleeding between these two groups (7% versus 6% respectively, P = ns). However, high bleeding rates led to discontinuation of treatment arms where abciximab was used in combination with SK.
The SPEED (Strategies for Patency Enhancement in the Emergency Department) trial randomized patients (n = 323) to either abciximab only (0.25 mg/kg bolus followed by a 12 h infusion of 0.125 μ g/kg/min), reteplase only (two 10 mg boluses) or both (reteplase being given as a double bolus of 2.5–15mg).33 The rate of TIMI-3 flow with abciximab in combination with two 5 mg boluses of reteplase was 54% at 90 minutes. This compared with a rate of 47% in those given full-dose reteplase only. The rate of major bleeding was numerically higher with combination therapy compared with reteplase alone (9.8% versus 3.7%, P = 0.11).
In the INTRO-AMI (Integrellin and Low-Dose Thrombolytics in Acute Myocardial Infarction) study, eptifibatide was administered as a single (180 μg/kg) or double bolus (180 then 90μg/kg or 180 then 180 μg/kg separated by 90 minutes), followed by an infusion of 1.33 or 2.00 μg/kg/ min for 72 h in combination with 25 or 50 mg of alteplase.34 The rate of TIMI-3 flow at 60 minutes was higher in those who received 50 mg of alteplase with eptifibatide 180 followed by 90 μ g/kg bolus and a 1.33 μg/kg/min infusion compared to those receiving full-dose alteplase alone (56% versus 40%, P = 0.04). Rates of major bleeding and intra-cranial hemorrhage were again numerically higher in the group who received combination therapy (11% versus 6% and 3% versus 2% respectively; P = ns for both).
Studies with clinical endpoints
Following the potentially encouraging results seen in the angiographic pilot studies, randomized trials with clinical endpoints have investigated the potential of combining a Gp IIb/IIIa receptor antagonist with a fibrinolytic agent (Fig. 32.2).
Figure 32.2 Clinical outcomes in the ASSENT-3,46 GUSTO-V,47 and HERO-248 trials: (a) mortality; (b) reinfarction.
The ASSENT-3 (Assessment of the Safety and Efficacy of a New Thrombolytic Regimen) trial randomized patients (n = 6095) within six hours of onset of STEMI to one of three treatment arms: full-dose tenecteplase and enoxaparin, half-dose tenecteplase and UFH plus abciximab or full-dose tenecteplase and UFH (control group). Compared with the control group, patients in the abciximab group had a significant reduction in the primary combined endpoint of death, reinfarction or refractory ischemia at 30 days (11.1% versus 15.4%, P < 0.0001), although there was no difference in mortality rates. Major bleeding (excluding intracranial hemorrhage) was increased (4.3% versus 2.2%, P < 0.005) and in those over 75 years old there was an approximately threefold increase (13.3% versus 4.1% respectively, P = 0.0002).
The GUSTO-V (Global Use of Strategies to Open Occluded Arteries) trial randomized patients (n = 16588) with STEMI presenting within six hours of onset to either full-dose (two 10 U bolus doses) or half-dose reteplase (two 5 U bolus doses) plus full-dose abciximab.35 Although there was a reduction in the reinfarction rate at 30 days with combination therapy (7.4% versus 8.8% with reteplase alone, P < 0.001), overall mortality was similar (5.9% for reteplase alone versus 5.6% for reteplase plus abciximab, P = 0.43) and remained so at one year (8.4% in both groups).36 In addition, there were again concerns over increased bleeding in those treated with combination therapy, with an increase in moderate to severe bleeding (excluding intracranial hemorrhage, 4.6% versus 2.3%; P < 0.001) and a trend towards an increase in intracranial hemorrhage in those over 75 years old (2.1% versus 1.1%; P = 0.07).
The recently presented Facilitated Intervention with Enhanced Reperfusion to Stop Events (FINESSE) trial has helped to shed some light on the role of combining abciximab with a fibrinolytic agent. However, it is important to note that the trial was principally designed to evaluate whether facilitated PCI (with upstream delivery of abciximab with or without reteplase) was superior to primary PCI (with in-lab abciximab). Patients (n = 2452) with an estimated time to the catheterization laboratory of between one and four hours were randomized in a 1:1:1 fashion to upfront abciximab-facilitated primary PCI, half-dose reteplase/abciximab-facilitated PCI, or primary PCI with in-lab abciximab. Consistent with a recent meta-analysis,37 the principal finding was that facilitated PCI provided no benefit and was associated with increased bleeding compared with standard primary PCI. There was no difference in the primary endpoint in the abciximab-only group compared to the combination group (9.8% versus 10.7% respectively, P = ns) but there was an increased incidence of TIMI major or minor bleeding (10.1% versus 14.5%, P = 0.008).
The use of abciximab in conjunction with half-dose reteplase or tenecteplase should be avoided in STEMI patients aged greater than 75 years due to an increased risk of intracranial hemorrhage (Class III, Level B).
Gp IIb/IIIa receptor antagonists in patients undergoing primary PCI
Primary PCI achieves TIMI-3 flow in the infarct-related artery in approximately 90% of patients. However, despite restoration of normal epicardial flow, flow within the downstream microvasculature and hence to the ischemic myocardium often remains impaired (the so-called “no reflow” phenomenon).38–40 This may be a key determinant of long-term outcome as diminished microvascular flow is associated with increased cardiovascular complications and mortality.40,41
The role of Gp IIb/IIIa receptor antagonists in the setting of primary PCI has been evaluated in conjunction with ASA and UFH use. All trials referred to in the discussion below have evaluated the complementary role of Gp IIb/IIIa receptor antagonists in this context, unless otherwise stated.
Abciximab
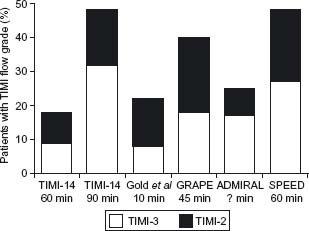
Abciximab is the most widely studied Gp IIb/ IIIa receptor antagonist. It has consistently been shown to increase rates of TIMI-3 flow in the infarct-related vessel, in a time-dependent manner (Fig. 32.3).
Figure 32.3 TIMI-2 and-3 flow rates in patients with acute MI treated with abciximab prior to angiography in the TIMI-14,32 Gold et al,95 GRAPE,96 ADMIRAL,43 and SPEED33 trials. The time intervals between administration of the abciximab bolus and angiography are shown. GRAPE, glycoprotein recepter antagonist patency evaluation.
The RAPPORT (ReoPro ® and Primary PTCA Organization and Randomized Trial) study was the first substantive study to investigate the effect of abciximab on a clinical endpoint following primary PCI (n = 409).42 It should be noted that stent use in this study was restricted to “bail-out” use only (i.e. abrupt vessel closure or dissection). The trial demonstrated a reduction in the 30-day rate of death, reinfarction or target vessel revascularization (TVR) in the abciximab group compared to placebo (11.2% versus 5.8%, P = 0.03).
The ADMIRAL (Abciximab before Direct Angioplasty and Stenting in Myocardial Infarction Regarding Acute and Long-Term Follow-up) study evaluated the effect of abciximab in patients (n = 300) with STEMI prior to primary PCI.43 A 41% reduction in the primary endpoint of death, reinfarction and TVR was observed at 30 days in the abcix-imab group (6.0% versus 14.6% in placebo group, P = 0.01), with a similar reduction at six months (7.4% versus 15.9%, P = 0.02). The investigators attributed the better outcome to higher levels of TIMI 3 flow in the target vessel before (16.8% versus 5.4%, P = 0.01) and after (95.1% versus 86.7%, P = 0.04) PCI. The association between postprocedural TIMI 3 flow and a lower incidence of major adverse cardiac event (MACE) at 30 days has also been observed in other studies.
The larger CADILLAC (Controlled Abciximab and Device Investigation to Lower Late Angioplasty Complications) trial randomized patients (n = 2082) with STEMI using a 2 × 2 factorial design to either abciximab or placebo, and either angioplasty or stenting.44 Patients were also given ASA, UFH and either clopidogrel or ticlopidine prior to going to the catheterization laboratory. The principal finding was a halving in the incidence of the composite endpoint (death, MI, stroke or TVR) at six months with stent insertion compared to angioplasty (20.0% versus 10.2%, P < 0.001). There was only a small additional benefit with abciximab (11.5% after stenting alone versus 10.2% after stenting plus abciximab, P = ns) although this may have been confounded by broad inclusion criteria and recruitment of a low-risk population coupled with relatively late administration of abciximab.
A meta-analysis of abciximab use in patients with STEMI undergoing primary PCI (n = 3266) demonstrated a reduction in the composite endpoint of death, reinfarction and need for TVR at 30 days (absolute RR 3.6%, relative RR 46%) and six months (absolute RR 3.2%, relative RR 20%).45 A further meta-analysis of 11 trials (n = 27 115) also demonstrated a reduction in short (30-day) and long-term (6–12 months) mortality (absolute RR 1% and 1.8% respectively; relative RR 29% for both) with abciximab use in the context of primary PCI.46 Neither demonstrated an increased incidence of intracranial hemorrhage although the latter analysis indicated an increase in the rate of major bleeding (absolute risk increase 1.2%, relative risk increase 68%, P < 0.001).
With regard to when best to administer a GP IIb/IIIa receptor antagonist in patients undergoing primary PCI, a meta-analysis of six small studies had suggested a greater prevalence of TIMI 2 or 3 flow in the infarct-related artery prior to PCI with early administration (41.7% versus 29.8%, P < 0.001).47 However, the FINESSE study (n = 2452) demonstrated no benefit of upstream (prior to catheter laboratory) administration of abciximab (or indeed upstream abciximb in combination with half-dose reteplase) compared to in-catheter laboratory administration.48
Tiro fiban and eptifibatide
Whilst tirofiban and eptifibatide share a common mechanism of action with abciximab, there have been no substantive trials with clinical end-points to assess their role in primary PCI, although small placebo-controlled studies suggest an increase in TIMI 2 or 3 flow in the infarct-related artery following early administration of tirofiban49 and eptifibatide50,51 respectively. The EVA-AMI trial, presented at the American Heart Association 2007 Scientific Sessions, randomized STEMI patients (n = 400) undergoing primary PCI to abciximab or eptifibatide. There were no significant differences in the primary endpoint (the rate of ST segment resolution 60 minutes following PCI) or the incidence of ischemic or bleeding events during the index admission. Similar results supporting the non-inferiority of tirofiban compared to abciximab were observed in the MULTISTRATEGY trial (n = 745).52
Patients with STEMI undergoing primary PCI should be treated with abciximab (Class I, Level A) as soon as possible prior to the procedure (Class I, Level B).
As an alternative, patients undergoing primary PCI may be treated with tirofiban or eptifibatide (Class IIb, Level B).
It should be emphasized that virtually all studies to date investigating the role of Gp IIb/IIIa receptor antagonists in STEMI patients undergoing primary PCI have been conducted prior to the routine administration of clopidogrel. Interestingly, in the CADILLAC study, which failed to show a significant benefit of abciximab, patients were pre-treated with a thienopyridine in addition to ASA. Whilst the study’ s broad entry criteria and subsequent recruitment of a relatively low-risk population may explain the lack of benefit with abciximab, it is possible that the routine administration of a thienopyridine diminished the relative efficacy of abciximab. The ON-TIME 2 study, showed a reduction in ST segment elevation at 60 minutes post-PCI (the primary endpoint) with tirofiban (initial 25 μg/kg bolus) compared with placebo.53 Patients in this study were also pretreated with ASA and clopidogrel (600 mg) and although not powered for clinical endpoints, there were encouraging trends in MACE at 30 days.
Taken in conjunction with the results from the HORIZONS AMI trial, discussed below, it is clear that further studies are required to clarify the role of Gp IIb/IIIa receptor antagonists in STEMI patients pretreated with ASA and clopidogrel who are undergoing primary PCI.
The coagulation cascade as at herapeutic target
Activation of the coagulation cascade plays an important role in thrombus formation during acute MI (see Fig. 32.1). Tissue factor, principally produced by monocytes within the plaque, binds to factor VII and initiates the extrinsic arm of the coagulation cascade, resulting in factor Xa generation. Factor Xa converts prothrombin to thrombin (factor IIa), the key enzyme responsible for the conversion of fibrinogen to its active form, fibrin. Multiple positive feedback mechanisms exist to enhance further production of factor Xa as well as promoting platelet activation and aggregation.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


