Valvular heart disease is prevalent and represents a significant contributor to cardiac morbidity and mortality. Several options for valve replacement exist, including surgical replacement and transcatheter valve implantation. Prosthetic valves lead to increased risk of thromboembolic disease; therefore, antithrombotic therapy after valve replacement is indicated. For patients with mechanical prostheses, indefinite vitamin K antagonist and antiplatelet therapy are the mainstays of treatment. There is no consensus regarding optimal antithrombotic therapy after bioprosthetic valve replacement, although vitamin K antagonist therapy of varying duration in addition to antiplatelet therapy is recommended by guidelines. Dual-antiplatelet therapy is commonly used after transcatheter valve implantation; however, alternative antithrombotic regimens are being studied. Further studies are needed to identify the optimal regimen, intensity, and duration of antithrombotic therapy after surgical bioprosthetic valve replacement and transcatheter valve implantation.
Background
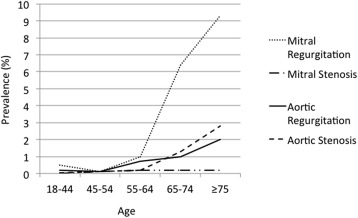
Valvular heart disease (VHD) is prevalent, particularly in the elderly, and remains a significant contributor to cardiac morbidity and mortality ( Figure 1 ). Surgical correction of VHD with either repair or replacement using a mechanical prosthesis or bioprosthesis represents the gold standard for treatment of symptomatic VHD. Less-invasive treatment of severe aortic stenosis with transcatheter aortic valve implantation (TAVI) has emerged as a common alternative to traditional surgery in patients with prohibitive or high surgical risk. In this review, we discuss antithrombotic therapy after surgical implantation and after TAVI.
Prosthetic Valve Thrombogenicity
The pathophysiology of thromboembolic (TE) complications in patients with prosthetic heart valves is complex, with endothelialization, valve characteristics, hemodynamics, platelet activation, and the coagulation cascade all playing major roles.
An intact vascular endothelium exhibiting antiplatelet, anticoagulant, and fibrinolytic properties is crucial to maintaining an appropriate balance between prothrombogenic and antithrombogenic states. Endothelialization of mechanical prostheses is often slow and incomplete. Bioprosthetic valves more closely mimic native heart valves as prosthetic leaflets are composed of biologic tissue such as extracellular matrix or pericardium. Valve surface characteristics such as material, design, size, and suture zone qualities all affect the extent or timing of endothelialization and represent important TE risk factors.
Bioprosthetic valves increase risk for TE complications, particularly during the first 3 months after implantation. This risk is attributed to lack of endothelialization of synthetic suture zones, suture knots, and valve leaflets in the early postoperative period. Pretreatment of bioprosthetic valves with fixatives (e.g., glutaraldehyde) improves valve stabilization, decreases antigenicity, and retards valve calcification; however, this may disrupt in vivo endothelialization and potentially further contribute to the risk of TE complications.
Position and composition of the prosthesis, intrinsic cardiac function, and the valve’s hemodynamic profile all contribute to thrombogenicity. Prosthetic valves are designed to avoid recirculation or stagnation zones as these are universally accepted as areas for thrombus formation. The thrombogenicity of bioprosthetic valves may also be dependent on the tissue type as comprehensive review data suggest that bovine pericardial valves may be less susceptible to valve thrombosis than porcine valves.
Activation of the coagulation cascade also plays an important role in valve thrombogenicity. The extrinsic (tissue factor) pathway may contribute to early postoperative thrombogenicity as endothelial trauma and subsequent release of tissue factor leading to factor VII activation are inevitable during surgery. Studies suggest that the extrinsic pathway plays a key role in both thrombosis and hemostasis.
The intrinsic (contact activation) pathway likely plays a larger role in valve thrombogenicity. The key event in activation of the intrinsic pathway is proteolytic activation of factor XII on a charged surface such as that of a mechanical valve leaflet. This leads to downstream activation of coagulation factors culminating in the generation of a fibrin clot. Studies suggest that the intrinsic pathway plays no role in hemostasis but does play an important role in thrombosis in the presence of an artificial compound.
Platelet activation due to high shearing forces resulting from turbulent blood flow, valve dynamics leading to cell trauma, and increased transvalvular pressure gradients increases valve thrombogenicity. Furthermore, activated platelets exposed to regional microrecirculation around a prosthetic valve are theorized to have increased opportunity for contact with (and subsequent activation of) other platelets.
Prosthetic Valve Thrombogenicity
The pathophysiology of thromboembolic (TE) complications in patients with prosthetic heart valves is complex, with endothelialization, valve characteristics, hemodynamics, platelet activation, and the coagulation cascade all playing major roles.
An intact vascular endothelium exhibiting antiplatelet, anticoagulant, and fibrinolytic properties is crucial to maintaining an appropriate balance between prothrombogenic and antithrombogenic states. Endothelialization of mechanical prostheses is often slow and incomplete. Bioprosthetic valves more closely mimic native heart valves as prosthetic leaflets are composed of biologic tissue such as extracellular matrix or pericardium. Valve surface characteristics such as material, design, size, and suture zone qualities all affect the extent or timing of endothelialization and represent important TE risk factors.
Bioprosthetic valves increase risk for TE complications, particularly during the first 3 months after implantation. This risk is attributed to lack of endothelialization of synthetic suture zones, suture knots, and valve leaflets in the early postoperative period. Pretreatment of bioprosthetic valves with fixatives (e.g., glutaraldehyde) improves valve stabilization, decreases antigenicity, and retards valve calcification; however, this may disrupt in vivo endothelialization and potentially further contribute to the risk of TE complications.
Position and composition of the prosthesis, intrinsic cardiac function, and the valve’s hemodynamic profile all contribute to thrombogenicity. Prosthetic valves are designed to avoid recirculation or stagnation zones as these are universally accepted as areas for thrombus formation. The thrombogenicity of bioprosthetic valves may also be dependent on the tissue type as comprehensive review data suggest that bovine pericardial valves may be less susceptible to valve thrombosis than porcine valves.
Activation of the coagulation cascade also plays an important role in valve thrombogenicity. The extrinsic (tissue factor) pathway may contribute to early postoperative thrombogenicity as endothelial trauma and subsequent release of tissue factor leading to factor VII activation are inevitable during surgery. Studies suggest that the extrinsic pathway plays a key role in both thrombosis and hemostasis.
The intrinsic (contact activation) pathway likely plays a larger role in valve thrombogenicity. The key event in activation of the intrinsic pathway is proteolytic activation of factor XII on a charged surface such as that of a mechanical valve leaflet. This leads to downstream activation of coagulation factors culminating in the generation of a fibrin clot. Studies suggest that the intrinsic pathway plays no role in hemostasis but does play an important role in thrombosis in the presence of an artificial compound.
Platelet activation due to high shearing forces resulting from turbulent blood flow, valve dynamics leading to cell trauma, and increased transvalvular pressure gradients increases valve thrombogenicity. Furthermore, activated platelets exposed to regional microrecirculation around a prosthetic valve are theorized to have increased opportunity for contact with (and subsequent activation of) other platelets.
Antithrombotic Therapy After Mechanical Valve Replacement
The American College of Chest Physicians (ACCP) and the American Heart Association/American College of Cardiology (AHA/ACC) provide similar recommendations regarding the use of anticoagulation after mechanical valve replacement ( Table 1 ), given the high risk of valve thrombosis and TE after mechanical valve replacement ( Figure 2 ). The mainstay of treatment is indefinite anticoagulation with a vitamin K antagonist (VKA). A meta-analysis from 1994 showed that no antithrombotic therapy, compared to VKA, was associated with a 4- to 9-fold increase in the rates of major systemic embolization (from 1.0 to 4.0), total TE (from 1.8 to 8.6), and valve thrombosis (from 0.2 to 1.8) per 100 patient-years. VKA therapy was also superior to aspirin monotherapy for total TE (1.8 [1.7 to 1.9] vs 7.5 [5.9 to 9.4] events per 100 patient-years) and valve thrombosis (0.2 [0.16 to 0.24] vs 1.0 [0.4 to 1.7] events per 100 patient-years). The risk of major bleeding in patients ranged from 1.2 to 2.6 events per 100 patient-years.
| Mechanical MVR | Mechanical AVR | Bioprosthetic MVR | Bioprosthetic AVR | TAVI | TMVI | ||
|---|---|---|---|---|---|---|---|
| ACCP Guidelines | Anticoagulation | Indefinite VKA, INR 2.5-3.5 (2C) | Indefinite VKA, INR 2.0-3.0 (1B) | 3-months VKA, INR 2.0-3.0 (2C) | No VKA if no other indication (2C) | No VKA if no other indication (2C) | NR |
| Antiplatelet | Indefinite ASA 50-100mg/day ∗ (1B) | Indefinite ASA 50-100mg/day ∗ (1B) | 3-months ASA 50-100mg/day (2C) | 3-months ASA 50-100mg/day (2C) | 3-months ASA 50-100mg/day + clopidogrel 75mg/day (2C) | NR | |
| AHA/ACC Guidelines | Anticoagulation | Indefinite VKA, INR 2.5-3.5 (I,B) | Indefinite VKA, INR 2.0-3.0 if no risk factors; INR 2.5-3.5 if risk factors † (I,B) | 3-months VKA, INR 2.0-3.0 (IIa,C) | 3-months VKA, INR 2.0-3.0 (IIb,B) | NR | NR |
| Antiplatelet | Indefinite ASA 75-100mg/day (I,A) | Indefinite ASA 75-100mg/day (I,A) | Indefinite ASA 75-100mg/day (IIa,B) | Indefinite ASA 75-100mg/day (IIa,B) | Indefinite ASA 75-100mg/day + 6-months clopidogrel 75mg/day (IIb,C) | NR |
∗ For those with low risk of bleeding.
† Risk factors = atrial fibrillation, prior thromboembolism, left ventricular dysfunction, hypercoagulable state.
Studies have shown the addition of aspirin to VKA therapy in patients with mechanical valves leads to reduction in risk of TE and mortality compared to VKA therapy alone (65% observed risk reduction in major systemic embolism or death in the aspirin plus VKA group). The addition of at least 75 to 100 mg/day of aspirin is therefore recommended in the current AHA/ACC and ACCP guidelines for all patients with mechanical valves at acceptable bleeding risk.
Attempts to broaden options for oral anticoagulation in patients with mechanical valves have been thus far unsuccessful. Investigators in the RE-ALIGN trial (Dabigatran versus warfarin in patients with mechanical heart valves) randomized patients who had undergone mechanical aortic or mitral valve replacement (MVR) within 7 days or ≥3 months to receive either dabigatran (150, 220, or 300 mg twice daily, based on renal function) or warfarin (international normalized ratio [INR] range 2.0 to 3.0 or 2.5 to 3.5 based on TE risk). The trial was terminated prematurely because of a significant excess of both TE events (hazard ratio [HR] 1.94 [0.64 to 5.86]) and bleeding events (HR 2.45 [1.23 to 4.86]) in patients in the dabigatran group.
Jaffer et al. provided an explanation of the results from RE-ALIGN based on observations from an ex vivo model that compared serum thrombin generation from patients on dabigatran or warfarin in the presence or absence of mechanical valve components. The authors reported that suppression of mechanical valve leaflet and sewing-ring associated thrombin generation required serum dabigatran concentrations that were much greater (≥400 ng/ml) than target trough concentrations used in the RE-ALIGN study (50 ng/ml). However, to maintain a concentration of dabigatran ≥400 ng/ml would be prohibitive because of increased risk for bleeding. The broader and more proximal effects of warfarin, which acts on both the intrinsic and extrinsic pathways of the clotting cascade, may be more effective at interrupting positive feedback loops that serve to amplify thrombin generation. Findings from RE-ALIGN have thus far precluded the use of non-VKA oral anticoagulants (NOACs) in patients with a mechanical prostheses, despite the absence of any randomized data with oral factor Xa inhibitors to date.
Newer mechanical prosthetic valves with reduced thrombogenicity may allow for less intense postoperative antithrombotic therapy. Puskas et al. randomized 375 patients undergoing aortic valve replacement (AVR) with the mechanical bileaflet On-X valve to low-range warfarin (INR goal 1.5 to 2.0) or standard-range warfarin (INR goal 2.0 to 3.0) after 3 months of standard-range warfarin therapy. All patients received low-dose aspirin. After an average follow-up of 3.8 years, patients in the low-range warfarin group experienced significantly less major (1.48% vs 3.26% per patient-year; p = 0.047) and minor (1.32% vs 3.41% per patient-year; p = 0.021) bleeding compared to standard-range warfarin. There was no between-group difference in the rates of stroke, transient ischemic attack, total neurologic events, or all-cause mortality. The investigators concluded that a goal INR of 1.5 to 2.0 in combination with low-dose aspirin could safely be maintained 3 months after AVR with the On-X mechanical prosthesis. Some clinicians, however, remain hesitant to adopt a less-intense level of anticoagulation pending additional experience.
Antithrombotic Therapy After Bioprosthetic Valve Replacement
The optimal antithrombotic regimen and duration of treatment after placement of a bioprosthetic valve are unclear. TE stroke rates after valve replacement with a bioprosthesis have been reported to range from 0.2% to 3.3% per year, with disproportionately greater risk for valves in the mitral position compared to valves in the aortic position. The risk of stroke with a bioprosthetic valve is lower than with a mechanical valve and is even lower (0.2% to 0.7% per year) for patients in normal sinus rhythm. The major clinical studies evaluating various antithrombotic regimens after bioprosthetic valve replacement are summarized in later sections and in Table 2 .
| Study | Design | Valve position: n | Mean f/u (mo) | Antithrombotic Therapy | Thromboembolic results | Bleeding results |
|---|---|---|---|---|---|---|
| Merie 2012 | Retro cohort | A: 4,075 | 78.8 pers-mo | W, ASA, W + ASA, no Rx | IRR 2.93 [1.54-5.55] for no W vs W | IRR 2.32 [1.28-4.22] for no W vs W |
| Brennan 2012 | Retro cohort | A: 25,656 | 3 | W, ASA, W + ASA, no Rx, other | RR 1.52 [0.35 to 0.76] for W + ASA vs ASA | RR 2.80 [2.18-3.60] for W + ASA vs ASA |
| Colli 2007 | Pro, rando | A: 75 | 3 | 3-mo W then ASA vs ASA alone | No difference in ischemic events | No difference in bleeding events |
| Brueck 2007 | Retro cohort | A: 288 | 12 | ASA vs no ASA | No difference in TE events | No difference in bleeding events |
| Aramendi 2005 | Pro, rando, multicenter | A:181 (94%), M:10 (5%), B: 2 (1%) | 6 | 3-mo VKA vs anti-plt | No difference in TE events | Significantly more bleeding w/ VKA |
| Sundt 2005 | Retro cohort | A:1151 (641 [56%] w/ CABG) | 3 | W vs no W; any anti-plt | HR 1.51 [0.66 to 3.46] for W vs no W | HR 1.49 [0.43 to 5.11] for W vs no W |
| Gherli 2004 | Pro, single-center, obs | A: 249 | > 6 | 3-mo ASA or W then ASA | No difference in cerebral ischemic events or survival | No difference in major bleeding events |
| Moinuddeen 1998 | Retro cohort | A: 185 | 53 | W, ASA, or no Rx | No difference in ischemic events | No difference in bleeding events |
| Goldsmith 1998 | Retro cohort | A: 145 (37 [26%] w/ CABG) | 25 | ASA | Rate of TE 0.7%/year | Rate of hemorrhage 0.4%/year |
| Heras 1995 | Retro cohort | A:424 (52%), M:326 (40%), B: 66 (8%) | 103.2 | W, ASA, DP, or no Rx | AC reduced risk of TE but anti-plt did not | AC increased risk of bleeding |
| Blair 1994 | Retro cohort | A: 378 (51%) M: 370 (49%) | 84 | W, ASA, no Rx | AVR: no diff. MVR: bleed w/ W=TE w/ ASA or no Rx | Hemorrhage w/ W > w/ ASA or no Rx |
| Nuñez 1984 | Retro cohort | M: 435 (57%) B: 333 (43%) | 32 | ASA (500mg QOD or 1000 mg QD) | TE rate 3% w/ high dose, 0.4% w/ low dose) | Not reported |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


