CHAPTER 21 Anterior Approach to Superior Sulcus Lesions
Henry K. Pancoast, a radiologist at the University of Pennsylvania, described a patient afflicted with a carcinoma of uncertain histologic origin occupying the extreme apex of the chest, associated with shoulder and arm pain, atrophy of the hand muscles, and Horner’s syndrome.1 This clinical entity has become known as Pancoast’s syndrome. Unknown to Pancoast, Tobias had already characterized the anatomic and clinical aspects of this lesion, correctly recognizing that the tumor was a peripheral lung cancer. Anatomically, the pulmonary sulcus refers to the costovertebral gutter extending from the first rib to the diaphragm. The superior pulmonary sulcus lies at the uppermost extent of this recess, as reviewed by Teixeira.2 Generally, it is understood that non–small cell lung carcinomas of this region are termed Pancoast tumors, and we refer to this association throughout. However, the term superior sulcus lesion encompasses other, more diverse etiologies, both benign and malignant. Furthermore, this definition has been expanded to include patients who do not have evidence of brachial plexus or stellate ganglion involvement. Chest wall involvement in this region might be restricted to involvement of the parietal pleura or could extend deeper to the upper ribs, vertebral bodies, or subclavian vessels according to Detterbeck.3 Invasion of the chest wall at or lower than the level of the second rib, or of the visceral pleura only, does not meet the criteria for a superior sulcus lesion. Additionally, Macchiarini and colleagues4 reported that a wide variety of superior sulcus lesions can result in Pancoast’s syndrome (Box 21-1); thus, a histologic diagnosis is required when the syndrome is encountered.
Box 21–1 Causes of Pancoast’s Syndrome
Adapted from Arcasoy SM, Jett JR. Superior pulmonary sulcus tumors and Pancoast’s syndrome. N Engl J Med 1997;337:1370.
PRESENTATION
Superior sulcus lesions of non–small cell histology account for less than 5% of all bronchial carcinomas, as reported by Ginsberg and associates.5 These tumors may arise from either upper lobe and tend to invade parietal pleura, endothoracic fascia, subclavian vessels, brachial plexus, vertebral bodies, or the first rib. However, their clinical features are influenced by their location.
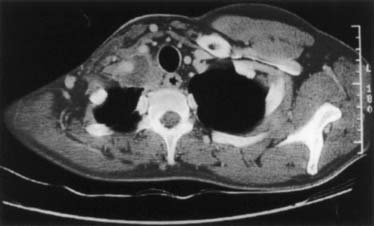
Tumors located anterior to the anterior scalene muscle may invade the platysma and sternocleidomastoid muscles, the external and anterior jugular veins, the inferior belly of the omohyoid muscle, the subclavian and internal jugular veins including major branches, and the scalene fat pad (Fig. 21-1). They invade the first intercostal nerve and the first rib more frequently than the phrenic nerve or superior vena cava. Patients usually complain of pain distributed to the upper anterior chest wall.
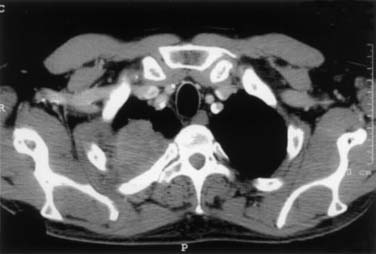
Tumors located between the anterior and middle scalene muscles may invade the anterior scalene muscle (phrenic nerve lying on its anterior aspect); the subclavian artery including primary branches, except the posterior scapular artery; and the trunks of the brachial plexus and middle scalene muscle (Fig. 21-2). These tumors manifest with signs and symptoms related to the compression or infiltration of the middle and lower trunks of the brachial plexus (e.g., pain and paresthesia radiating to the shoulder and upper limb).
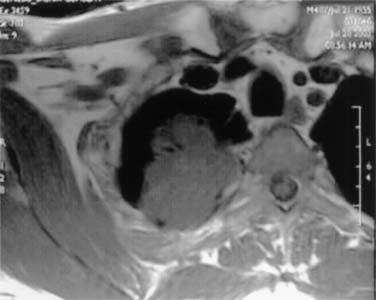
Tumors lying posterior to the middle scalene muscles are usually located in the costovertebral groove and invade the nerve roots of T1, the posterior aspect of the subclavian and vertebral arteries, the paravertebral sympathetic chain, the inferior cervical (stellate) ganglion, and the prevertebral muscles. Some of these posterior tumors can invade transverse processes (Fig. 21-3) or extend to the vertebral bodies (only those abutting the costovertebral angle or extending into the intraspinal foramen without intraspinal extension may be resected).
PREOPERATIVE STUDIES
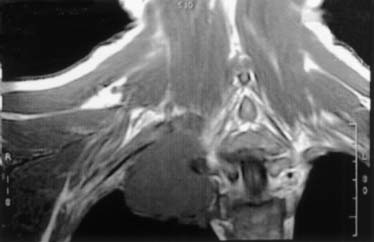
Neurologic examination, magnetic resonance imaging (MRI), or electromyography may delineate the tumor’s extension to the brachial plexus, phrenic nerve, or epidural space. Vascular invasion is evaluated by venous angiography, subclavian arteriography, Doppler ultrasonography (cerebrovascular disorders may contraindicate resection of the vertebral artery), or MRI (Fig. 21-4). Additional MRI is mandatory if the workup suggests tumor encroachment into the intervertebral foramina, to rule out invasion of the extradural space (Fig. 21-5).
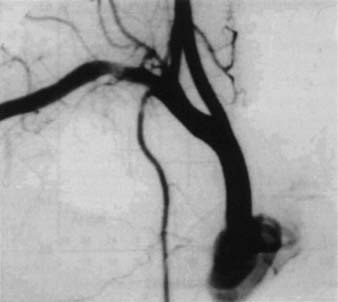
Figure 21–4 Angiography illustrating a massive tumoral invasion of the intrascalenic left subclavian artery.
TREATMENT
Tumors of the superior sulcus are deemed universally and rapidly fatal lesions. For many years, it was believed that these tumors were not amenable to surgery, until Chardack and MacCallum6 successfully performed a lobectomy and chest wall excision followed by radiation therapy. Five years later, Shaw and colleagues7 approached superior sulcus tumors with preoperative radiation therapy (30 to 45 Gy in 4 weeks, including the primary tumor, mediastinum, and supraclavicular region) followed by surgical resection. This radiosurgical approach shortly became the standard treatment, yielding better disease control and survival than other treatments. More recently, Ginsberg and colleagues5 provided evidence that en bloc resection of the tumor combined with external radiation (preoperative, postoperative, or both) must be considered the standard therapeutic approach for superior sulcus tumors. The goal of resection is the complete removal of the upper lobe in continuity with the invaded ribs, transverse processes, subclavian vessels, T1 nerve root, upper dorsal sympathetic chain, and prevertebral muscles.
In 1999, we reviewed various surgical approaches for treating superior sulcus lesions.8 In general, superior sulcus lesions not invading the thoracic inlet are completely resectable through the classic posterior approach of Shaw and colleagues.7 However, the posterior approach does not allow direct, safe visualization, manipulation, or complete oncologic clearance of all anatomic structures of the thoracic inlet. Superior sulcus lesions extending to the thoracic inlet should be resected by an anterior transcervical approach according to our previous description.9
ANTERIOR TRANSCERVICAL TECHNIQUE
One-lung anesthesia with measurements of urine output and body temperature are necessary. An arterial line opposite to the primary lesion, and at least two venous lines for volume expansion should be used. The patient is supine with the neck hyperextended and the head turned away from the involved side. A bolster behind the shoulder elevates the operative field. The skin preparation extends from the mastoid downward to the xiphoid process and from the midaxillary line laterally to the contralateral midclavicular line medially. An L-shaped cervicotomy incision is made, including a vertical pre-sternocleidomastoid incision carried horizontally below the clavicle up to the deltopectoral groove (Fig. 21-6). To increase the exposure, the interception between the vertical and horizontal branches of the L-shaped incision is lowered to the level of the second or third intercostal space, depending on tumor extent. The incision is then deepened with cautery. The sternal attachment of the sternocleidomastoid muscle is divided. The cleidomastoid muscle, along with the upper digitations of the ipsilateral pectoralis major muscle, is scraped from the clavicle. A myocutaneous flap is then folded back, providing full exposure of the neck and cervicothoracic junction.
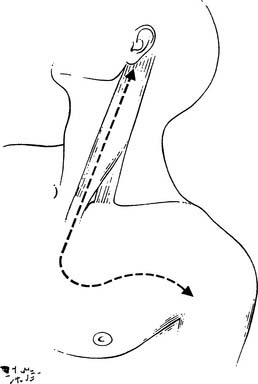
Figure 21–6 Anterior transcervical approach.
(As described in Dartevelle PG, Chapelier AR, Macchiarini P, et al. Anterior transcervical-thoracic approach for radical resection of lung tumors invading the thoracic inlet. J Thorac Cardiovasc Surg 1993;105:1025.)
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree