Anomalies of Pulmonary Venous Return
Carlos M. Mery
James J. Gangemi
Irving L. Kron
Anomalies of pulmonary venous return form a spectrum of embryologically related congenital heart defects that have in common the failure of the pulmonary veins to unite normally with the left atrium. The lesion may involve anomalous connection of all four pulmonary veins with the systemic venous circulation (total anomalous pulmonary venous return, TAPVR) or abnormal drainage of at least one but not all pulmonary veins into the systemic venous circulation (partial anomalous pulmonary venous return, PAPVR).
TOTAL ANOMALOUS PULMONARY VENOUS RETURN
Historical Aspects
The earliest description of TAPVR was given by Wilson in 1798. In 1942, Brody presented an autopsy series of patients with this anomaly. Muller at UCLA is credited with the initial attempt of surgical treatment of TAPVR in 1951. He described a closed operation consisting of a side-to-side anastomosis between the common pulmonary vein trunk and the left atrial appendage. In 1956, Lewis, Varco, and associates at the University of Minnesota Minneapolis, MN, reported the first successful total correction of TAPVR using hypothermia and inflow occlusion. The same year, Burroughs and Kirklin described their experiences with surgical correction using cardiopulmonary bypass (CPB). The introduction of deep hypothermic circulatory arrest by Barratt-Boyes and colleagues in the early 1970s was a major advance toward accomplishing surgical repair because this technique provides a bloodless operative field. Further advances in early diagnostic modalities, neonatal intensive care, including the availability of extracorporeal membrane oxygenation (ECMO), and pediatric cardiac surgical anesthesia, as well as an awareness of the merits of early surgical intervention, have contributed to the excellent results with TAPVR repair reported recently by many centers.
Embryology
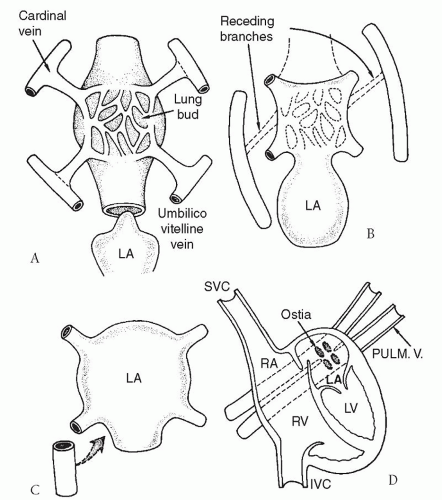
The respiratory system develops as an evagination from the foregut at 26 days. The venous plexus from the early lung buds drains into the anterior cardinal and umbilico vitelline veins, both of which are part of the splanchnic (systemic) venous system. The anterior cardinal veins give rise to the right and left superior vena cavae (SVC), the coronary sinus, and the azygos vein. The umbilico vitelline veins form the inferior vena cava (IVC) and the portal vein. In normal circumstances, the common pulmonary vein develops as an outpouching from the dorsal left atrial wall, eventually fusing with the pulmonary venous plexus at 28 to 30 days. Shortly thereafter, the anterior cardinal and umbilico vitelline venous channels normally undergo involution (Fig. 97.1). Failure of the common pulmonary vein to unite with the pulmonary venous plexus leads to persistence of these embryonic pulmonary venous-to-systemic venous anastomoses, yielding total anomalous pulmonary venous drainage into right atrial tributaries. In some instances, abnormal leftward displacement of the developing atrial septum results in anomalous connection of all four pulmonary venous ostia directly to the right atrium.
Anatomic Defects and Classification
The common underlying anatomic defect in all cases of TAPVR involves anomalous drainage of the entire pulmonary venous circulation into the right atrium, either directly, or via the SVC, IVC, or coronary sinus. The pulmonary venous blood may drain into the systemic venous circulation through a single common channel or by multiple portals of entry. An interatrial communication, usually manifested as a patent foramen ovale (PFO) or secundum atrial septal defect (ASD), is mandatory for shunting of partially oxygenated blood to the left heart.
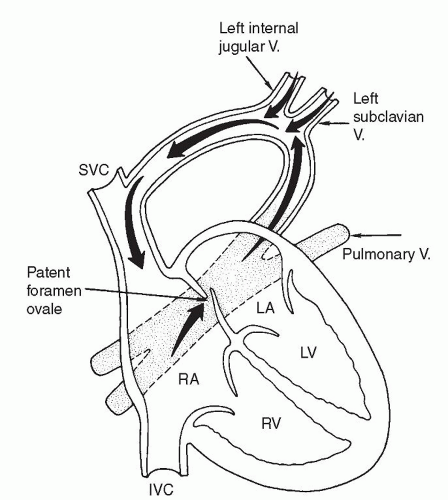
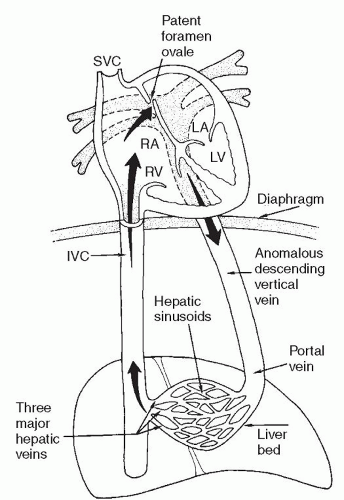
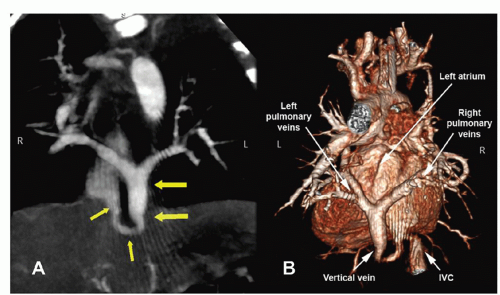
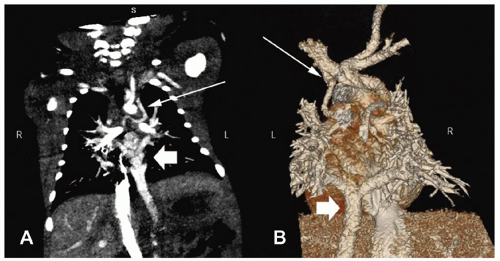
There are several classification schemes for TAPVR. The most commonly used system is the one described in 1957 by Darling and associates. This four-tier system is based on the site of pulmonary venous drainage into the systemic circulation. In type I, or supracardiac TAPVR, all four pulmonary veins form a horizontal common pulmonary venous confluence behind the left atrium that gives rise to a vertical vein that drains into a supracardiac systemic vein (innominate vein, SVC, or azygos vein) (Fig. 97.2). The most common configuration involves an ascending left vertical vein or persistent left SVC that drains into the innominate vein. Type II, or cardiac TAPVR, is characterized by total pulmonary venous drainage into a markedly dilated coronary sinus (Fig. 97.3) or, less commonly, directly into the right atrium. Type III, or infracardiac TAPVR, involves a more vertical pulmonary venous confluence giving rise to a descending vertical vein that travels through the esophageal hiatus to below the diaphragm, where it most often makes an anomalous connection with the portal vein, one of its branches (Fig. 97.4), or the ductus venosus. In such cases, pulmonary venous blood returns to the right atrium by way of the IVC. Type IV, or mixed TAPVR, comprises all mixed defects with connections at different levels.
According to most series, a supracardiac connection is the most common TAPVR variant (45% to 60%), whereas the cardiac and infracardiac types are encountered somewhat less frequently (15% to 30% each) and the mixed type is the rarest (5% to 10%). In general, the infracardiac variants of TAPVR are usually associated with significant obstruction of the anomalous draining vein due to the length of the venous channel and the resistance created in the hepatic portal system. Supracardiac variants are significantly obstructed approximately half of the time while cardiac variants rarely present with significant obstruction. Alternate classification systems have used the embryologic origin of the anomalous connection, the length
of the draining veins, and the degree of obstruction between the pulmonary venous and systemic venous pathways. The most complete system, proposed by Herlong and associates as part of the Congenital Heart Surgery Nomenclature and Database Project, describes the anatomic variant, the presence or absence of obstruction, and the type of obstruction (extrinsic or intrinsic compression). Whereas the majority of TAPVR cases are isolated anomalies, the lesion occasionally coexists with other cardiac and extracardiac congenital malformations. TAPVR, especially in autopsy series, has been diagnosed concomitantly with a variety of other acyanotic and cyanotic heart defects, including patent ductus arteriosus, valvular atresia and stenosis, ventricular septal defect, transposition of the great arteries, tetralogy of Fallot, double-outlet right ventricle, and common atrioventricular canal. In addition, there is a well-known association between TAPVR and the heterotaxy syndrome, which includes visceral heterotaxy, isomerism, dextrocardia, and splenic abnormalities (asplenia, polysplenia, hyposplenia). In some series, up to 30% of patients with TAPVR have heterotaxy syndrome.
of the draining veins, and the degree of obstruction between the pulmonary venous and systemic venous pathways. The most complete system, proposed by Herlong and associates as part of the Congenital Heart Surgery Nomenclature and Database Project, describes the anatomic variant, the presence or absence of obstruction, and the type of obstruction (extrinsic or intrinsic compression). Whereas the majority of TAPVR cases are isolated anomalies, the lesion occasionally coexists with other cardiac and extracardiac congenital malformations. TAPVR, especially in autopsy series, has been diagnosed concomitantly with a variety of other acyanotic and cyanotic heart defects, including patent ductus arteriosus, valvular atresia and stenosis, ventricular septal defect, transposition of the great arteries, tetralogy of Fallot, double-outlet right ventricle, and common atrioventricular canal. In addition, there is a well-known association between TAPVR and the heterotaxy syndrome, which includes visceral heterotaxy, isomerism, dextrocardia, and splenic abnormalities (asplenia, polysplenia, hyposplenia). In some series, up to 30% of patients with TAPVR have heterotaxy syndrome.
Pathophysiology
The physiologic consequences of TAPVR depend largely on the presence and magnitude of pulmonary venous obstruction. In those cases with no significant obstruction, TAPVR functions as a large left-to-right shunt. Pulmonary blood flow is greatly increased, and right ventricular overload occurs as a result of both the pulmonary venous and systemic circulations returning to the right atrium. Consequently, pulmonary hypertension, pulmonary edema, right ventricular enlargement, and congestive heart failure supervene. Cyanosis results from the mixing of fully oxygenated pulmonary venous blood with desaturated systemic venous blood. A compensatory right-to-left shunt through a PFO or an ASD is mandatory for survival because this allows shunting of partially oxygenated blood to the left atrium for systemic distribution. Although left atrial and left ventricular volumes are generally small due to shunting, these chambers are rarely hypoplastic.
Obstruction to pulmonary venous blood flow in TAPVR may occur as a result of extrinsic compression, intrinsic luminal narrowing of the vein, a restrictive communication at the atrial level (e.g., a small PFO or ASD), or a combination of
these. The infracardiac TAPVR is associated with significant obstruction in 80% to 100% of patients. Usually, the descending vertical vein is obstructed at its junction with the portal vein or ductus venosus. In addition, resistance to blood flow through the hepatic sinusoids in the setting of a closed ductus venosus produces a functional obstruction. Supracardiac TAPVR variants may be obstructed due to a “viselike” compression of the vertical vein between the left pulmonary artery anteriorly and the left mainstem bronchus posteriorly.
these. The infracardiac TAPVR is associated with significant obstruction in 80% to 100% of patients. Usually, the descending vertical vein is obstructed at its junction with the portal vein or ductus venosus. In addition, resistance to blood flow through the hepatic sinusoids in the setting of a closed ductus venosus produces a functional obstruction. Supracardiac TAPVR variants may be obstructed due to a “viselike” compression of the vertical vein between the left pulmonary artery anteriorly and the left mainstem bronchus posteriorly.
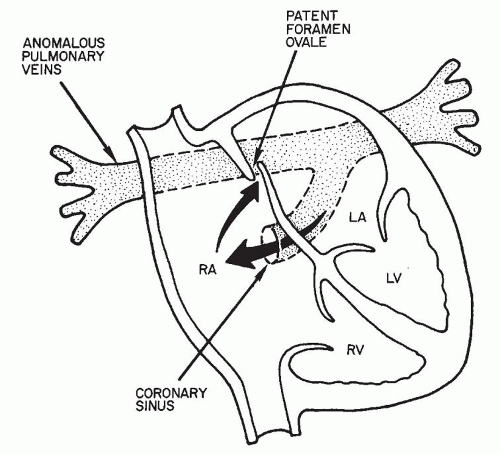 Fig. 97.3. Pathologic anatomy of cardiac-type total anomalous pulmonary venous return via the coronary sinus. (LA left atrium; LV, left ventricle; RA, right atrium; RV, right ventricle.) |
In patients with pulmonary venous obstruction, significant pulmonary edema results from the increased hydrostatic pressure in the pulmonary capillary bed proximal to the obstruction. The degree of cyanosis will depend on the balance between systemic and pulmonary blood flow. Patients with significant obstruction will have profound cyanosis due to a significantly decreased amount of oxygenated blood returning to the heart and mixing with the much larger volume of systemic deoxygenated blood. Pulmonary hypertension is usually present and most severe in patients with significant obstruction. In those patients, pulmonary artery pressures approach or exceed systemic pressures.
Diagnosis
Signs and Symptoms
The condition of infants with TAPVR is determined largely by the presence and degree of obstruction to pulmonary venous return. Nonobstructed TAPVR often eludes diagnosis at birth and in the early neonatal period. Months later, patients may present with the gradual onset of tachypnea, dyspnea, congestive heart failure, and mild cyanosis. Often, subtle complaints of feeding difficulties and failure to thrive may be the only clues of congenital heart disease in these children. Liver congestion with hepatomegaly and cardiomegaly along with a prominent right ventricular impulse are consistent features.
Cardiac examination may be unimpressive. There may exist either a gallop or a faint diastolic murmur, most often a result of increased flow across the tricuspid valve. Other findings are the hallmarks of increased pulmonary blood flow, such as a systolic ejection murmur over the left second interspace and a prominent fixed S2 component.
In contrast, infants with obstructed TAPVR may present in extremis within hours to days after birth. These infants are profoundly cyanotic and show severe congestive heart failure. Hypotension and metabolic acidosis are frequently present. Findings on cardiac examination are inconsistent, but the right-sided cardiac chambers may be of normal size. The liver may also be of normal span and free of congestion if the ductus venosus remains patent and thus is able to divert pulmonary venous blood directly into the inferior vena cava.
Chest X-Ray
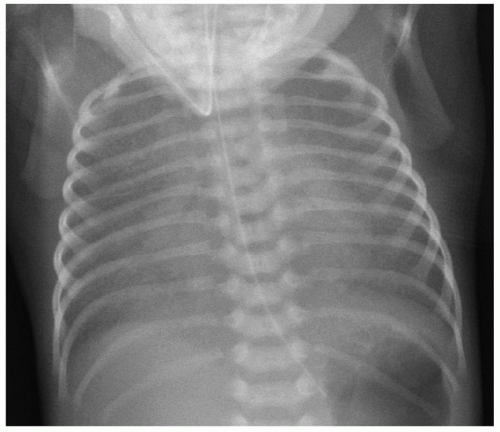
Chest X-ray features of TAPVR are again dependent on the presence or absence of significant pulmonary venous obstruction. Nonobstructed TAPVR is characterized by a normal-sized heart with increased pulmonary vascularity, possibly a distinct pulmonary artery silhouette, and occasional enlargement of the right cardiac silhouette. The classic X-ray images of a “snowman” or “figure-of-eight” in supracardiac connection are rarely appreciated before 6 months of life. In cases of obstructed TAPVR, the cardiac silhouette is usually of normal size but there is marked engorgement of the pulmonary vasculature and diffuse interstitial infiltrates in a reticular pattern indicative of severe pulmonary edema due to venous obstruction (Fig. 97.5).
Echocardiography
Modern two-dimensional echocardiographic technology and techniques have revolutionized the noninvasive diagnosis of TAPVR. In unstable, critically ill neonates, echocardiography prevents any undue delay in diagnosis and surgical therapy. Since the 1980s, echocardiography has become the mainstay for preoperative diagnosis and classification of TAPVR. Two-dimensional echocardiography with Doppler color-flow
mapping is extremely accurate and reliable in diagnosing TAPVR, delineating the exact drainage pattern of each individual pulmonary vein, detecting the presence and degree of obstruction, and identifying any concomitant cardiac malformations. The absence of pulsatile pulmonary venous blood flow and dilation of the venous confluence or the vertical vein indicate obstruction. The vertical vein is usually well visualized and can be followed to its site of drainage in the systemic circulation.
mapping is extremely accurate and reliable in diagnosing TAPVR, delineating the exact drainage pattern of each individual pulmonary vein, detecting the presence and degree of obstruction, and identifying any concomitant cardiac malformations. The absence of pulsatile pulmonary venous blood flow and dilation of the venous confluence or the vertical vein indicate obstruction. The vertical vein is usually well visualized and can be followed to its site of drainage in the systemic circulation.
Computerized Tomography and Magnetic Resonance Imaging
Contrast computerized tomography (CT) and magnetic resonance imaging (MRI) may be useful in patients in which echocardiography fails to delineate the exact anatomy. These modalities can provide important information, particularly in cases of mixed TAPVR or complex anatomy (Figs. 97.6 and 97.7). The downsides of these diagnostic studies (the time needed to obtain them and the administration of contrast) must be weighed against the information to be obtained, especially in patients with obstructed TAPVR that need urgent surgical intervention.
Cardiac Catheterization and Angiocardiography
In the current era, cardiac catheterization is rarely required and is mainly of historical interest. It should be reserved only for cases in which echocardiographic, CT, and MRI findings are inconsistent with the clinical course. The precise site of anomalous pulmonary venous connection is identified by a “step-up” in oxygen saturation in a systemic vein, whereas the exact course of pulmonary venous drainage is delineated during the levophase of selective pulmonary arteriography.
It is common for the blood in all four cardiac chambers to have equal or similar oxygen saturations, which reflects the mixing of pulmonary and systemic venous circulations. TAPVR and transposition of the great arteries are the only two conditions in which the oxygen saturation of blood in the main pulmonary artery is equal to or greater than that in the aorta. Right ventricular and pulmonary artery pressures are frequently elevated in TAPVR but are virtually always so in the presence of obstruction. The adequacy of the PFO in terms of its ability to shunt partially oxygenated blood to the left side of the heart may be estimated by the difference between right atrial pressure and pulmonary capillary wedge or left atrial pressure. The detection of a transatrial pressure gradient suggests a restrictive foramen ovale. Balloon atrial septostomy may be used to temporarily relieve significant obstruction at the atrial level if immediate surgical intervention is not possible.
Preoperative Management and Timing of Surgical Intervention
Infants presenting with obstructed TAPVR represent a surgical emergency. An intensive resuscitation period should be followed by expeditious surgical intervention. Immediate interventions include endotracheal intubation and mechanical ventilatory support. Pulmonary vasoconstriction should be avoided. Although pulmonary vasodilatory measures such as administration of 100% oxygen and hyperventilation to a partial arterial pressure of carbon dioxide (PaCO2) of <30 mmHg have been traditionally used, it is unclear whether they provide any significant benefit. Metabolic acidosis should be treated with sodium bicarbonate or tromethamine infusions. Function of the
failing heart is augmented with the administration of inotropic and diuretic agents. Maintenance of ductal patency with a prostaglandin E1 infusion may also be of some physiologic benefit.
failing heart is augmented with the administration of inotropic and diuretic agents. Maintenance of ductal patency with a prostaglandin E1 infusion may also be of some physiologic benefit.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree