Infective endocarditis (IE) is a life-threatening disease. Considered the gold standard for the diagnosis of IE, the modified Duke criteria rely on echocardiographic findings to satisfy its major criterion. Echocardiography is an invaluable tool in the evaluation of patients with suspected IE but suffers from certain limitations. For example, it cannot differentiate vegetation from clot, or between infected and noninfected vegetation, and may miss vegetation and periannular extensions in the presence of prosthetic material. Therefore, alternative cardiac imaging modalities are needed. Nuclear imaging, particularly 18 F-fluorodesoxyglucose positron emission tomography–computed tomography (CT), is becoming increasingly popular in the evaluation of patients for IE and has shown promise in diagnosing valvular and device-related IE when echocardiography results were inconclusive. Other techniques such as radiolabeled leukocyte scintigraphy and single-photon emission computed tomography with or without CT are less well studied, however. Cardiac CT angiography is also evolving as a powerful supplementary tool to echocardiography for the detection of perivalvular complications of IE and for preoperative evaluation of coronary anatomy. The combination of cardiac CT angiography and echocardiography is superior to either test alone in the diagnosis of IE and its complications. Although brain magnetic resonance imaging may impact prognosis and clinical management by identifying cerebral emboli in patients with IE, the role of cardiac and abdominal magnetic resonance imaging is less clear. In conclusion, with these additional diagnostic tools at our disposal, the diagnosis of IE may be achieved in a more timely and accurate manner to secure better clinical outcomes.
Infective endocarditis (IE) is a life-threatening disease with a mortality rate of 22% to 27%. The modified Duke criteria remain the gold standard for the diagnosis of IE. A major criterion is evidence of endocardial involvement provided by echocardiographic findings such as oscillating intracardiac masses, abscesses, new partial dehiscence of a prosthetic valve, or new valvular regurgitation. The sensitivity of transthoracic echocardiography and transesophageal echocardiography (TEE) for detecting valvular vegetation is around 60% and 95%, respectively, for native valves and 50% and 90%, respectively, for prosthetic valves, whereas the reported specificity is 90% for both techniques. However, echocardiography can be falsely negative if performed early in the course of infection when morphological changes have not yet occurred, cannot differentiate infected from noninfected vegetation or clot from vegetation on intracardiac leads, and may miss vegetation and periannular extensions (abscesses, mycotic aneurysms) because of acoustic shadowing by prosthetic material. Therefore, alternative less-invasive diagnostic techniques are needed.
Nuclear Imaging
Nuclear imaging techniques are becoming important supplementary methods in the evaluation for IE. 18 F-fluorodesoxyglucose (18F-FDG) positron emission tomography–computed tomography (PET-CT), radiolabeled leukocyte scintigraphy, and single-photon emission computed tomography (SPECT) with or without CT have all been studied as adjunctive tools in this setting.
PET-CT is well established in the field of oncology for the diagnosis and staging of cancer. Just over a decade ago, it was shown that PET-CT could provide evidence of endocardial inflammation in the setting of IE well before morphological changes such as vegetation developed. Occasionally, prosthetic valve IE (PVIE) was discovered only after imaging with PET-CT after negative TEEs.
The physiological uptake of 18F-FDG by normal myocardium depends on patient age, fasting time, blood glucose level, and dietary carbohydrate consumption. Cardiac uptake of 18F-FDG is variable in cases of IE and depends on the timing of the study in relation to the start of antibiotic therapy. The likelihood of false-positive results increases with a greater interval time between antimicrobial initiation and acquisition of images. As well, variable foci of diffuse physiological uptake of 18F-FDG in healthy myocardium can lead to false-positive results. The combination of PET and CT over PET alone improves localization of focal uptake and aids with the differentiation of pathologic uptake from normal physiological uptake.
PET-CT can identify foci of peripheral embolization and metastatic infection in up to 45% of cases of IE, including splenic and renal infarcts, septic arthritis, osteomyelitis, pulmonary septic emboli, and muscle abscesses. Because of high physiological uptake of 18F-FDG in the brain, gingiva, heart, and kidneys, PET-CT has limited ability to detect septic emboli to these tissues and organs. Identification of extracardiac manifestations of IE is highest in organs with low physiological 18F-FDG uptake such as the eyes, liver, spleen, intestines, vertebrae, bones, joints, orthopedic hardware, and muscle with a sensitivity and specificity of 87% to 100% and 80% to 97%, respectively. Moreover, if PET-CT is performed more than 6 days after the diagnosis of IE, the sensitivity of detecting any emboli improves to 91% from 79%. Occasionally, PET-CT can reveal the original source of bacteremia, identify concomitant infections, and identify possible related malignancies, particularly of the bowel.
PET-CT has proved most useful in the evaluation of left-sided PVIE ( Figure 1 ). Data regarding its use in native valve and right-sided IE are sparse. In 1 prospective trial, results of PET-CT led to reclassification of 90% of cases initially classified as possible IE with the modified Duke criteria and provided a definitive diagnosis in 95% of cases, either rejected or definite IE. PET-CT also outperformed echocardiography in identifying abscesses and pseudoaneurysms. In other studies, PET-CT findings led to a modification of management in up to 35% of cases, including antibiotic duration, surgical referral, joint aspiration or lavage, and avoidance of unnecessary device extraction. The number needed to investigate to find a clinically important lesion that would change management was 7.
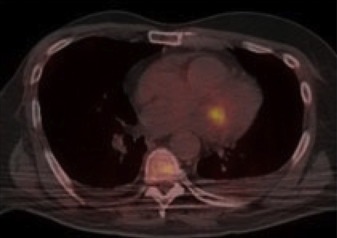
PET-CT boasts a sensitivity of 39% to 93% and a specificity of 80% to 93% compared to the modified Duke criteria for the diagnosis of PVIE, but its accuracy is reduced with native valve IE (NVIE). Compared to echocardiography, sensitivity and specificity with PET-CT were 85% and 100%, respectively, for PVIE, whereas sensitivity was lower and even dismal at 0% for NVIE in a single study.
PET-CT can aid the diagnosis of cardiac implantable electronic device (CIED) infections ( Figure 2 ). In patients with suspected CIED-related IE and negative echocardiography, PET-CT improved the accuracy of the modified Duke criteria (sensitivity 63% to 100%, specificity 86% to 93%) by reclassifying possible to definite IE cases. When CIED infection is suspected, it is important to distinguish between infection of the superficial skin, pocket site, and the intracardiac leads because removal of the device is not necessary for superficial skin infection, whereas extraction of intracardiac leads is occasionally difficult and can be associated with complications. In a prospective study of patients undergoing evaluation with PET-CT for possible CIED infection, a positive result indicative of a deep infection led to device extraction in 70% to 95% of cases, of which all were later confirmed to be true infection by a positive culture, clinical status, and/or intraoperative findings. The sensitivity and specificity for infection of the superficial skin, pocket site, and intracardiac leads were 100% and 100%, 87% and 100%, and 31% and 63%, respectively. Whether new antibacterial envelopes now available with some new CIED which were shown to reduce CIED infection rate (e.g., TYRX, Medtronic, Monmouth Junction, New Jersey) also affect the detection rate of positive IE cases with PET-CT still remains to be seen. Of note, even when traditional diagnostic tools such as blood cultures and TEE are negative, PET-CT can still identify CIED lead infection by contributing important information regarding active infection-derived inflammation to the anatomic data derived from CT or TEE.

PET-CT is limited in assessing for infection of vascular grafts and stents. In patients with cancer with prosthetic vascular grafts who underwent PET-CT for assessment of their malignancy, diffuse and persistent 18F-FDG uptake was observed in 92% of noninfected grafts. Consequently, a positive result may be misinterpreted as a vascular graft infection if caution is not exercised.
Some experts have proposed revising the modified Duke criteria to include PET-CT as a novel criterion. Indeed, adding abnormal 18F-FDG uptake around a prosthetic valve as a major criterion increased sensitivity of the modified Duke criteria from 70% to 97%, while retaining specificity by reclassifying possible to definite cases of IE.
Its limitations include poor performance in NVIE and that most studies cited used PET-CT after inconclusive echocardiography. Studies from our group and others have shown low real-world TEE use in suspected IE, and the late acquisition of TEE may delay the application of PET-CT with decreasing sensitivity.
Data are limited regarding the use of radiolabeled leukocyte scintigraphy in the diagnosis of IE. In 1 prospective study of patients (n = 42) with suspected PVIE and inconclusive TEEs, leukocyte scintigraphy identified perivalvular complications or poor outcomes under medical treatment in 9 patients and excluded the presence of an abscess in 3 patients. A greater proportion of patients with a positive scan underwent cardiac surgery than those with a negative result (43% vs 11%). The sensitivity and specificity of technetium-99m ( 99m Tc)–labeled leukocyte scintigraphy are 64% and 100%, respectively.
Gallium-67 ( 67 Ga) citrate SPECT for the diagnosis of IE has only been described in case studies. Prosthetic aortic valve IE was identified by 67 Ga citrate SPECT in 2 patients with Gram-positive bacteremia of unknown origin, whereas SPECT/CT detected infection of a bioprosthetic aortic valve in 1 patient and identified CIED lead infection in another after a negative TEE.
In patients with confirmed NVIE and PVIE, predominantly aortic and mitral, 99m Tc-hexamethylpropyleneamine oxime–labeled white blood cells (HMPAO-WBC) with SPECT/CT had a sensitivity and specificity of 90% and 100%, respectively, in diagnosing IE compared to the final diagnosis at 12-month follow-up. Septic embolisms to the lung, spleen, and spine were detected in 41% of patients. Reclassification to definite IE occurred in 11 of 88 patients with negative or inconclusive echocardiograms. In a study of patients with CIED infections, 99m Tc-HMPAO-WBC with SPECT/CT exhibited a sensitivity and specificity of 94% and 100%, respectively, for the detection and localization of CIED-associated infection compared to the final diagnosis at the end of follow-up. In addition to outperforming echocardiography (sensitivity 63%, specificity 87%), it helped to define the extent of infection (pocket vs lead) and revealed metastatic complications (osteomyelitis, mediastinitis, lung infection, and vascular graft infection). False-negative results occurred with vegetation <6 mm in size, infection with Candida and Enterococcus , and previous receipt of antibiotics. Results of 99m Tc-HMPAO-WBC with SPECT/CT did not influence clinical management such as device removal or antimicrobial therapy, however.
Computed Tomography
Electrocardiography (ECG)–gated cardiac CT angiography (CCTA) with new-generation scanners (≥64 slices) allows rapid scanning of the heart and great vessels, requires minimal patient cooperation, and has high diagnostic accuracy for identifying coronary disease. Because of the “motion-free” image generated by ECG gating, CCTA can provide valuable information on valvular abnormalities including the number, thickness, and opening and closing of the leaflets and the presence and location of valve calcification. As with echocardiography, CCTA recognizes endocardial inflammation only after morphological changes have developed. CCTA with new-generation multislice scanners that have improved temporal resolution and submillimeter spatial resolution may have the advantage over echocardiography in patients with more extensive calcifications of valves and in the assessment of deep tissue or perivalvular extent of the infection (abscess, pseudoaneurysms; Figures 3-5 ). Furthermore, despite blooming artifacts from metallic implants, CCTA may be especially useful in PVIE where acoustic shadowing limits TEE sensitivity. Gated and nongated CT are able to identify vegetation on right-sided valves while simultaneously detecting pulmonary septic emboli.

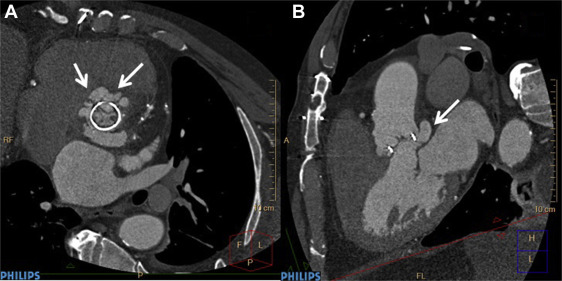
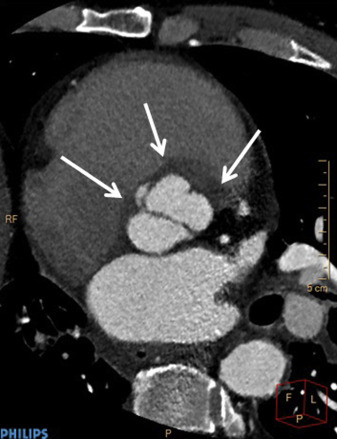
The accuracy of CT in diagnosing IE and its complications is generally good. In a small study of patients with primarily left-sided IE (89% native, 11% prosthetic), the mean vegetation size seen on multislice CT was 7.1 mm (range 2 to 22 mm), which correlated well with TEE findings. Small vegetation (≤4 mm) tended to be missed. Preoperative CT correctly identified all 10 paravalvular abscesses, pseudoaneurysms, or fistula seen intraoperatively, awarding it a sensitivity and specificity of 100% for the diagnosis of these complications. However, it missed all 4 mitral valve leaflet perforations <2 mm. Compared to TEE, multislice CT had a sensitivity and specificity of 97% and 88%, respectively, on a per-patient–based analysis, and 97% and 95%, respectively, on per-valve–based analysis for detecting valvular abnormalities. Compared to intraoperative findings, sensitivity and specificity were 96% and 97%, respectively, on a per-valve–based analysis. In a similar study, ECG-gated cardiac CT was positive in 93% of patients with TEE-confirmed prosthetic aortic valve IE. Although TEE was superior in revealing valvular dehiscence and vegetation, ECG-gated cardiac CT was superior in uncovering pseudoaneurysms, suggesting these 2 techniques may be complementary. Congruency with intraoperative findings was optimal when results of CT and TEE were combined. It is important to note that with ECG-gated reconstruction of a CCTA image, a single phase of the cardiac cycle is usually analyzed, therefore limiting diagnostic accuracy of CCTA for detection of a sessile structure which may be detected only during part of diastole or of systole. Moreover, TEE with Doppler has the advantage of identifying physiological or pathologic leaks or stenosis often related to valve infection. In another small study of patients with mostly native aortic valve IE of whom all underwent cardiac surgery, multidetector CT exhibited a sensitivity and specificity of 100% and 88%, respectively, for detecting aortic perivalvular pseudoaneurysms and 71% and 100%, respectively, for identifying vegetation compared to intraoperative findings. Both sensitivity and specificity increased to 100% for vegetation >10 mm in size.
In addition to facilitating a diagnosis of IE, ECG-gated CCTA with a modern scanner can serve as a reliable alternative to catheter-based invasive angiography by simultaneously providing a noninvasive preoperative assessment of coronary anatomy.
Computed Tomography
Electrocardiography (ECG)–gated cardiac CT angiography (CCTA) with new-generation scanners (≥64 slices) allows rapid scanning of the heart and great vessels, requires minimal patient cooperation, and has high diagnostic accuracy for identifying coronary disease. Because of the “motion-free” image generated by ECG gating, CCTA can provide valuable information on valvular abnormalities including the number, thickness, and opening and closing of the leaflets and the presence and location of valve calcification. As with echocardiography, CCTA recognizes endocardial inflammation only after morphological changes have developed. CCTA with new-generation multislice scanners that have improved temporal resolution and submillimeter spatial resolution may have the advantage over echocardiography in patients with more extensive calcifications of valves and in the assessment of deep tissue or perivalvular extent of the infection (abscess, pseudoaneurysms; Figures 3-5 ). Furthermore, despite blooming artifacts from metallic implants, CCTA may be especially useful in PVIE where acoustic shadowing limits TEE sensitivity. Gated and nongated CT are able to identify vegetation on right-sided valves while simultaneously detecting pulmonary septic emboli.
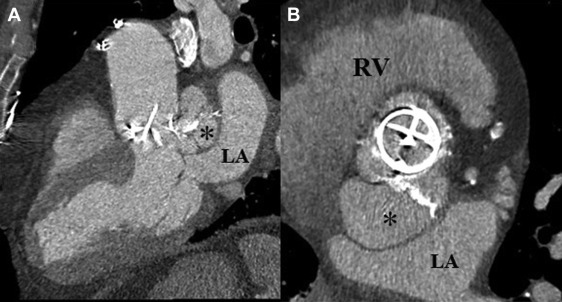
The accuracy of CT in diagnosing IE and its complications is generally good. In a small study of patients with primarily left-sided IE (89% native, 11% prosthetic), the mean vegetation size seen on multislice CT was 7.1 mm (range 2 to 22 mm), which correlated well with TEE findings. Small vegetation (≤4 mm) tended to be missed. Preoperative CT correctly identified all 10 paravalvular abscesses, pseudoaneurysms, or fistula seen intraoperatively, awarding it a sensitivity and specificity of 100% for the diagnosis of these complications. However, it missed all 4 mitral valve leaflet perforations <2 mm. Compared to TEE, multislice CT had a sensitivity and specificity of 97% and 88%, respectively, on a per-patient–based analysis, and 97% and 95%, respectively, on per-valve–based analysis for detecting valvular abnormalities. Compared to intraoperative findings, sensitivity and specificity were 96% and 97%, respectively, on a per-valve–based analysis. In a similar study, ECG-gated cardiac CT was positive in 93% of patients with TEE-confirmed prosthetic aortic valve IE. Although TEE was superior in revealing valvular dehiscence and vegetation, ECG-gated cardiac CT was superior in uncovering pseudoaneurysms, suggesting these 2 techniques may be complementary. Congruency with intraoperative findings was optimal when results of CT and TEE were combined. It is important to note that with ECG-gated reconstruction of a CCTA image, a single phase of the cardiac cycle is usually analyzed, therefore limiting diagnostic accuracy of CCTA for detection of a sessile structure which may be detected only during part of diastole or of systole. Moreover, TEE with Doppler has the advantage of identifying physiological or pathologic leaks or stenosis often related to valve infection. In another small study of patients with mostly native aortic valve IE of whom all underwent cardiac surgery, multidetector CT exhibited a sensitivity and specificity of 100% and 88%, respectively, for detecting aortic perivalvular pseudoaneurysms and 71% and 100%, respectively, for identifying vegetation compared to intraoperative findings. Both sensitivity and specificity increased to 100% for vegetation >10 mm in size.
In addition to facilitating a diagnosis of IE, ECG-gated CCTA with a modern scanner can serve as a reliable alternative to catheter-based invasive angiography by simultaneously providing a noninvasive preoperative assessment of coronary anatomy.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


