Chapter 96 Advances in Catheter Ablation of Primary Ventricular Fibrillation
Ventricular fibrillation (VF) is a potentially lethal cardiac rhythm disorder and is the most frequent cause of sudden cardiac death (SCD). It can complicate the course of any cardiomyopathy or occur unexpectedly in patients who do not have structural heart disease. It is often initiated by ventricular premature depolarization at a critical coupling interval (primary VF) or by ventricular tachycardia (VT) that further degenerates into fibrillatory activity (secondary VF). The Purkinje-myocardium junction plays a key role in triggering ectopy and in sustaining re-entry in the early stages of VF.1 Perpetuation of VF is thought to be mediated either by one or several periodical sources, intramyocardial and intra-Purkinje re-entry, or by multiple interacting wavelets.2,3 Both mechanisms have been observed during VF in humans.3
Premature Ventricular Contractions Initiating Ventricular Fibrillation
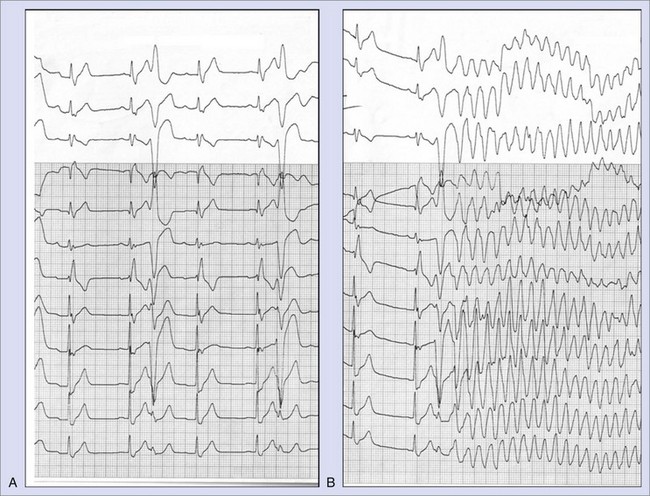
The vulnerable period during which a premature ventricular beat can provoke VF encompasses the end of ventricular repolarization. Conditions that lengthen QT increase the risk of VF; however, in idiopathic VF, the QT interval is normal. In patients with no apparent heart disease, short-coupled premature ventricular contractions (PVCs) occurring within 20 to 40 ms of the peak of the T wave have been documented to initiate VF.4 Although the culprit PVCs initiating VF may be monomorphic in patients with normal hearts, PVCs are often polymorphic in patients with structural heart disease (Figures 96-1 and 96-2).
The role of the Purkinje network in triggering PVCs leading to VF in humans has been evidenced by mapping PVCs in survivors from VF and SCD.5 All patients from this initial description had normal hearts and were diagnosed as having idiopathic VF. Conventional mapping for the earliest activation during premature beats revealed that PVCs originated from the Purkinje network in 12 of 16 patients, the others having PVCs originating from the myocardial tissue.5 Further studies confirmed this finding: In a series of 27 patients with idiopathic VF, PVCs originated from the Purkinje network in 23 and from the right ventricular outflow tract (RVOT) myocardial tissue in 4.6 Three studies in the literature reported ablation of VF triggers in a total of 17 patients with ischemic cardiomyopathy. All mapped PVCs arose from the Purkinje system, mainly from the left ventricle.7–9 In 4 patients with a history of VF and long QT syndrome (LQTS), 3 had PVCs triggered by Purkinje tissue and 1 from RVOT, whereas in 3 patients with Brugada syndrome, only 1 had Purkinje-initiated ectopic beats.10 Eight patients with malignant early repolarization had a total of 26 different ectopic beat morphologies, 10 arising from Purkinje tissue and 16 from the myocardium.11 Finally, isolated reports of Purkinje PVCs initiating VF after aortic valve repair, in the context of an acute coronary syndrome, in infiltrative cardiac amyloidosis, in coronary artery disease, and in chronic myocarditis are found in the literature.12 In a minority of cases, the culprit PVCs originate from myocardial tissue, mostly from the RVOT area. This finding, however, seems to be more frequent in patients with Brugada syndrome.
Catheter Ablation for Ventricular Fibrillation
Indications
In patients with multiple episodes of VF occurring despite medical therapy, catheter ablation directed at VF triggers can be attempted. Current guidelines state that “ablation of Purkinje fiber potentials may be considered in patients with ventricular arrhythmia storm consistently provoked by PVCs of similar morphology” as a class IIb level of evidence C recommendation.13
When clusters of VF occur in a patient, investigations should initially search for secondary modifiable arrhythmogenic conditions such as myocardial ischemia, electrolyte imbalance, QT prolongation caused by medication, substance abuse or medication abuse or toxicity, hypothermia and rare conditions, for example, infiltrative cardiac disorders, or cardiac masses or tumors. In the absence of a reversible cause, a drug trial is indicated and should be tailored to the underlying pathology. In the absence of contraindications, patients with ischemic heart disease should receive β-blockers or amiodarone; patients with LQTS can be treated with β-blockers; and those with malignant early repolarization syndrome, idiopathic VF, and Brugada syndrome should be treated with quinidine.13–15 Implantable cardioverter-defibrillators (ICDs) should be implanted in all SCD survivors if no reversible cause is found to explain the event. If these strategies fail to control VF episodes, catheter ablation should be considered.
Electrocardiogram Morphology of Premature Ventricular Contractions
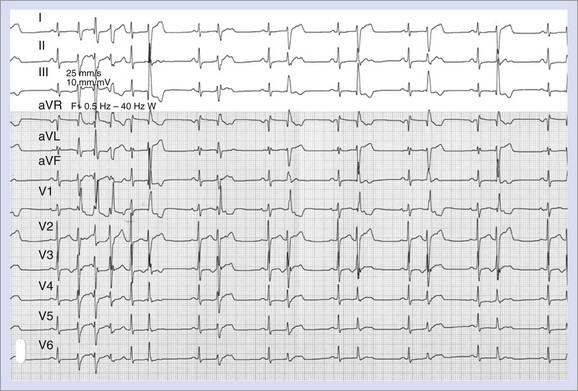
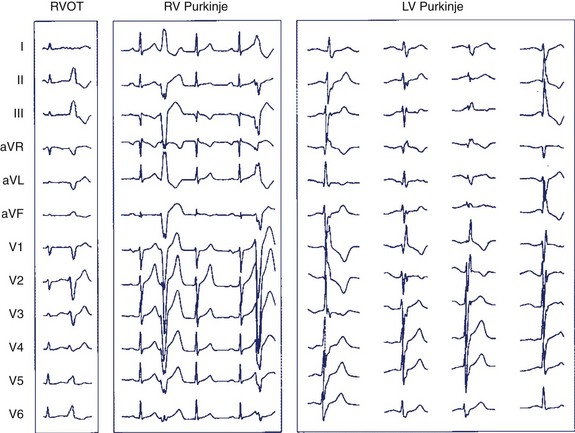
Twelve-lead ECG reveals important clues for the ablation procedure. First, it documents if PVCs are monomorphic or polymorphic. PVC negativity in V1 simulating a left bundle branch block pattern indicates origination from the right ventricle and indicates origination from the left ventricle when positive in V1 with a right bundle branch block appearance. PVC polymorphism can be seen in 85% of patients. The arborization of the Purkinje network makes it possible for the influx to take different routes, and the exit point may vary from one PVC to another, causing subtle changes in the morphology of ectopic beats. These changes are dependent on the extent of the Purkinje arborization and, therefore, are more evident when PVCs originate from the left ventricle and more subtle when origination is from the right, as the right-sided Purkinje network has fewer ramifications than the left-sided Purkinje network (Figure 96-3). PVCs arising from the Purkinje network typically display a relatively narrow QRS and a sharp onset, more abrupt as the PVC arises from the proximal Purkinje or fascicules and become more slurred distally. Right Purkinje ectopic beats are typically wider in duration than is left Purkinje ectopy.5 PVCs arising from myocardial tissue display wider QRS (145 ms vs. 126 ms for Purkinje ectopies in one study, P = .04) and a slurred uptake (Figures 96-4 and 96-5).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree