Chapter 131 Adult Congenital Cardiac Surgery
Patients with congenital heart disease require life-long surveillance. The prevalence of congenital heart disease is approximately 1% of all live births.1 Tremendous advances in the diagnosis and treatment of congenital heart lesions have resulted in improved survival rates, and 85% to 90% of infants born with congenital heart disease will reach adulthood.2 There are over 1 million adults with congenital heart disease (ACHD) in the United States, and this population grows by approximately 5% each year.3,4
The challenges of caring for ACHD were recognized as early as 1973, when Dr. Joseph Perloff published an article describing the pediatric congenital cardiac patient who becomes a postoperative adult.5 However, it was not until 1990 at the 22nd Bethesda Conference of the American College of Cardiology that the care of adults with congenital heart disease was formally recognized as a subspecialty of cardiology care.6 In 2001, the 32nd Bethesda Conference attempted to address the changing profile of adults living with congenital heart disease by developing guidelines for resource allocation.2
EPIDEMIOLOGY
There are now more adults than children living with congenital heart disease, and the population continues to grow. A recent study conducted in Quebec, Canada, reported that the prevalence of adults with congenital heart disease was 4.09 per 1000 for the year 2000, which was an 85% increase compared with 1985. This compares with an only 22% increase in the prevalence of children with congenital heart disease during the same time period.7 The current growth of the adult congenital heart population is an indication of clinical advances made in the late 1980s and 1990s. This presents several unique challenges to the health care providers who care for these patients.
In 2001, the 32nd Bethesda Conference developed guidelines for resource allocation and program development directed at ACHD. The model for delivery of care focuses on classification of congenital heart defects into different levels of complexity (Box 131-1), with referral to specialized adult congenital disease centers for increasing levels of complexity. More than half of the population with ACHD is believed to be at increased risk for sudden cardiac death, need for reoperation, and other severe complications. This report estimated that 15% of patients with ACHD have complex disease and require regular follow-up at a specialized center for adult congenital heart disease, and an additional 38% have moderate disease requiring periodic follow-up in a specialized center.2 The British Cardiac Society Working Party published a similar document addressing the care for the “grown-up congenital heart” (GUCH) population.8 The consensus of both documents is that all patients with the exception of those with simple heart lesions should receive specialized ACHD care.
STRUCTURED PROGRAMS FOR ADULTS WITH CONGENITAL HEART DISEASE
Over the past decade, there has been an increase in the number of centers with specialized teams to care for ACHD patients. These centers should have a multidisciplinary team committed to the life-long care of patients. There are many challenges in creating and maintaining a successful team of specialized ACHD providers. Health care providers who specialize in the care of these patients have various levels of experience in ACHD diagnosis and management. Although they are committed to the care of their patients, they may not have been trained in this subspecialty.9 The best ACHD programs benefit from the multidisciplinary collaboration of specialists not only in congenital cardiac disease but also in reproductive and obstetric care, nephrology, hepatology, hematology, pulmonary and psychiatric support. From a cardiac perspective, it is essential to have experienced clinicians with expertise in imaging, electrophysiology, interventional catheterization, congenital cardiac surgery, cardiac anesthesia, advanced heart failure management, and transplantation medicine. The organizational structure should be regionally coordinated, so that regional centers caring for ACHD can easily consult and refer to the specialized programs.10
Lapses in medical care for ACHD result in adverse outcomes. In a study of 158 adult patients referred to one adult congenital heart center, a lapse of medical care, defined as an interval of more than 2 years, occurred for 99 patients (63%). The median duration of lapse of care was 10 years. Patients with lapse of care were 3.1 times more likely to require urgent cardiac interventions (P = .003).11 Emergencies in the ACHD are of particular concern. In one multicenter study of hospital admissions of ACHD, 63% of emergent admissions required cooperation with another specialized department.12 An additional challenge that faces the ACHD population is lack of insurance coverage. Congenital heart disease patients older than 17 years and without private insurance are at higher risk of being admitted via the emergency room.13
A critical volume of patients is needed to maintain expertise in the various disciplines required in the care of ACHD patients. Adult congenital heart surgery should be performed only by surgeons who are experienced in all aspects of congenital heart surgery.14 The choice of location for surgery should depend not only on the surgeon but also on the multidisciplinary team’s support for management of adult comorbidities. Adult cardiac surgical procedures are performed at children’s hospitals with excellent results.15
SURGICAL INDICATIONS IN ADULTS WITH CONGENITAL HEART DISEASE
The American College of Cardiology and the American Heart Association recently developed guidelines for managements of ACHD, which include recommendations for surgical interventions.16 The decision to proceed with surgery in the ACHD patient is multifactorial. The goals of prolonging survival and improving quality of life are paramount. Previously established measures of physical functioning (such as New York Heart Association [NYHA] class) are difficult to assess in the ACHD patient. Many patients who have lived with the sequelae of cardiac disease since birth do not perceive themselves as “limited” despite objectively measured, markedly diminished exercise capacity.17 ACHD may not recognize subtle changes in their exercise tolerance. Additionally, there is a common perception that surgical palliation of a congenital heart defect in infancy or childhood was a cure. Therefore, by the time that patients are formally evaluated, ventricular dysfunction may be severe and irreversible.18
A subset of ACHD patients are referred for primary surgical correction. The principal reason for this is late diagnosis—for example, patients with atrial septal defects or aortic coarctation. Patients may also be referred as adults for a condition that was previously considered inoperable, or because they are from a geographic region without local surgical congenital heart expertise, or because they have had balanced systemic and pulmonary blood flows despite complex lesions. Patients with unrepaired tetralogy of Fallot and pulmonary atresia have a poor prognosis; survival beyond the 5th decade is rare.19 Another important consideration in the decision to recommend surgery is the presence of comorbidities. The potential benefits of surgery must be weighed against the risk of operative death and postoperative morbidities.
PREOPERATIVE EVALUATION
Cardiac magnetic resonance imaging (MRI) is increasingly used to quantify ventricular volumes, function, and vascular anatomy.20 Gadolinium-enhanced contrast MRI may be used for assessment of myocardial viability. Cardiac computed tomography is an alternative for patients who have contraindications to cardiac MRI, and it provides excellent coronary imaging.21
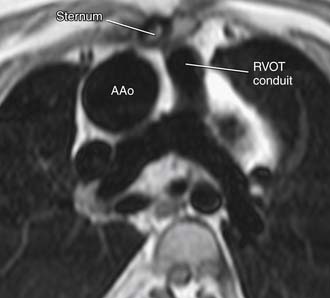
Imaging can also be very helpful to determine the anatomic relationships of certain vascular structures, such as the relationship of the aorta to the sternum (Fig. 131-1). This is particularly important for repeat sternotomy, which may be associated with inadvertent aortic entry. Preoperative duplex ultrasonography of the femoral and iliac arteries and veins reveals the status of the patency of these vessels in ACHD patients with previous interventions. This is essential for patients who need urgent peripheral cannulation for cardiopulmonary bypass.
Cardiac catheterization is often required to determine the cardiac hemodynamics and whether there are associated lesions that should be addressed in the catheterization laboratory.22 Coronary angiography should be considered when there are risk factors, such as angina, noninvasive evidence of ischemia, reduced systemic ventricular function, a history of myocardial infarction, or prior coronary surgery. Angiography can also be used for adults with transposition of the great arteries who have undergone an arterial switch procedure with coronary translocation.23 The consensus is that selective coronary angiography should be performed in ACHD patients referred for surgery who are older than 40 years.24 As many as 33% of ACHD patients may have an asymptomatic coronary artery anomaly that is discovered with coronary angiography—an important finding prior to surgery.25 Exercise capacity should be examined with objective exercise testing.26
An intensive electrophysiologic evaluation of the preoperative ACHD patient is essential. The incidence of arrhythmias is highest in those patients with moderate or severe ACHD complexity. For example, as many as a third of adults with repaired tetralogy of Fallot develop symptomatic atrial tachycardia,27 about 10% develop high-grade ventricular arrhythmias,28 and about 5% require permanent pacemaker placement for sinus node dysfunction or atrioventricular block. Additionally, an increasing number of adults with repaired tetralogy of Fallot receive an implantable cardioverter defibrillator for treatment of ventricular tachyarrhythmia.29
The importance of a multisystem approach to the ACHD patient during the preoperative evaluation cannot be overemphasized. In a recent retrospective analysis of over 1100 ACHD patients, 9% had moderate or severe renal dysfunction with a reported threefold increase in mortality.30 Lung function can be significantly diminished in the ACHD patient by a previous thoracotomy and the resultant scoliosis and restrictive lung disease. ACHD patients with cyanosis are at risk for multisystem derangement, including abnormal hematologic indices, coagulopathy, nephropathy, and hepatic dysfunction.
ACHD patients and their families should be educated on the potential benefits and possible risks of the surgical procedure. Some ACHD patients have emotional problems from previous childhood interventions, and their concerns must be addressed preoperatively. They may have a deleterious body image because of surgical scars.31 A dedicated discussion to the options regarding surgical entry, including possible techniques to minimize further scarring, is often helpful.
PERIOPERATIVE MANAGEMENT
The physiology of the ACHD patient is often complex, and an experienced cardiac anesthetist must be responsible for the care of the patient from the preinduction period through to the postoperative management. The experienced anesthetist is essential in managing the shifts in the vascular resistance of the systemic and pulmonary circulations, the dynamics of intracardiac shunting, and the intravascular volume status. Possible harmful hemodynamic effects related to each anesthetic agent must be considered.32 For example, spinal anesthesia can result in a reduction in preload and decreased pulmonary blood flow. It can also result in a drop in the systemic vascular resistance and an increase pulmonary-to-systemic shunting, thus worsening hypoxemia. Narcotics may contribute to an acute reduction in systemic vascular resistance during induction.
Coordination of the operating room team in planning and completing successful sternal reentry is essential. The great majority of surgeries performed on ACHD patients are reoperations. Reoperations are associated with a higher risk, particularly in patients with cyanosis, transposed great arteries (anterior aorta), pulmonary atresia, or poor ventricular function.33 In one report, early mortality for reoperation approached 10% for ACHD patients who underwent five sternotomies.34 The surgeon must recognize the relationships of cardiac structures in the ACHD patient, many of whom have greatly distorted anatomy, particularly when pericardial integrity has been lost because of previous surgical interventions. Cardiac MRI provides excellent visualization of the relationship of cardiac structures to the sternum (see Fig. 131-1). In some patients, there is no discernable space between the posterior table of the sternum and a cardiac structure.
Dilated or hypertensive right heart structures are at particular risk during repeat sternotomy. For example, patients with a systemic right ventricle, such as those with complete transposition of the great arteries who have undergone atrial switch repair (e.g., Mustard, Senning) have an enlarged anterior right ventricle that may be adherent to the posterior shelf of the sternum. Aneurysmal, thin-walled, right ventricular outflow tract patches and dilated ascending aortas may also be closely related to the sternum. The surgeon must be aware of the location of previously placed extracardiac conduits or shunts to avoid possible injury on sternal reentry. Another example is a massively dilated and thinned-out right atrium in a classic Fontan operation. It typically crosses the midline and abuts the sternum, and injury to it can propagate because the wall tends to be very thin. Additionally, a preoperative knowledge of the coronary anatomy is helpful to avoid inadvertent coronary injury. Certain congenital diagnoses have a higher incidence of anomalous coronary origins and courses, such as tetralogy of Fallot, where the left anterior descending coronary artery crosses the right ventricular outflow tract in at least 5% of cases.35
Management of Bleeding during Repeat Sternotomy
Strategies to minimize postoperative blood loss include the following:
ACHD patients who have depressed ventricular function and limited cardiac reserve do not tolerate large volume shifts well, and meticulous attempts must be made to achieve postoperative hemostasis. Patients with decreased ventricular function may need mechanical ventricular support (e.g., ventricular assist device, extracorporeal membrane oxygenation) for a limited time in the immediate postoperative period, and appropriate devices should be available during the surgical procedure in case the patient fails to wean from cardiopulmonary bypass.
Strategies to improve myocardial protection include the following:
POSTOPERATIVE MANAGEMENT
The surgical care must be performed in a setting with a structured ACHD program that has a multidisciplinary team approach, and this is evident postoperatively. Nursing staff should be skilled in working with ACHD patients. The physiology of the lesions often dictates postoperative care. Avoidance of acidosis and maintenance of adequate oxygenation and systemic perfusion are paramount. Postoperative bleeding should be anticipated and controlled. Patients should be reexplored if bleeding is excessive after the first several postoperative hours. Recognition and treatment of arrhythmias must be prompt and successful to avoid hemodynamic instability. The majority of ACHD patients should return from the operating room with both atrial and ventricular temporary pacing wires, which may be used in determining the type of arrhythmia so as to direct therapy. Monitoring lines are also very useful: a transthoracic left atrial line, a right atrial line, or a pulmonary artery line can be left in the patient. The intensive care outcomes on adults operated on for congenial heart disease are favorable.36 For 2851 ACHD patients operated on at the Mayo Clinic from 1993 to 2006, the early mortality rate was 2.4%.34
The majority of ACHD patients have residual cardiac issues after repeat surgery, and they must be educated about the need for long-term follow-up. Several organizations are dedicated to improving the care of adults with congenital heart disease. The International Society for Adult Congenital Heart Disease was founded in 1994 with a mission to promote, maintain, and pursue excellence in the care of adults with congenital heart disease (www.isachd.org). The Adult Congenital Heart Association, a national nonprofit organization founded in 1988 by a group of ACHD survivors and their families, is dedicated to improving the quality of life and extending the lives of adults with congenital heart defects. More than 60 specialized clinics for adults with congenital heart disease are listed on their website (www.achaheart.org).