ACUTE RHEUMATIC FEVER
Acute rheumatic fever (ARF) is a multisystem disease resulting from an autoimmune reaction to infection with group A streptococcus. Although many parts of the body may be affected, almost all of the manifestations resolve completely. The exception is cardiac valvular damage (rheumatic heart disease [RHD]), which may persist after the other features have disappeared.
GLOBAL CONSIDERATIONS
 ARF and RHD are diseases of poverty. They were common in all countries until the early twentieth century, when their incidence began to decline in industrialized nations. This decline was largely attributable to improved living conditions—particularly less crowded housing and better hygiene—which resulted in reduced transmission of group A streptococci. The introduction of antibiotics and improved systems of medical care had a supplemental effect. Recurrent outbreaks of ARF began in the 1980s in the Rocky Mountain states of the United States, where elevated rates persist.
ARF and RHD are diseases of poverty. They were common in all countries until the early twentieth century, when their incidence began to decline in industrialized nations. This decline was largely attributable to improved living conditions—particularly less crowded housing and better hygiene—which resulted in reduced transmission of group A streptococci. The introduction of antibiotics and improved systems of medical care had a supplemental effect. Recurrent outbreaks of ARF began in the 1980s in the Rocky Mountain states of the United States, where elevated rates persist.
The virtual disappearance of ARF and reduction in the incidence of RHD in industrialized countries during the twentieth century unfortunately was not replicated in developing countries, where these diseases continue unabated. RHD is the most common cause of heart disease in children in developing countries and is a major cause of mortality and morbidity in adults as well. It has been estimated that between 15 and 19 million people worldwide are affected by RHD, with approximately one-quarter of a million deaths occurring each year. Some 95% of ARF cases and RHD deaths now occur in developing countries.
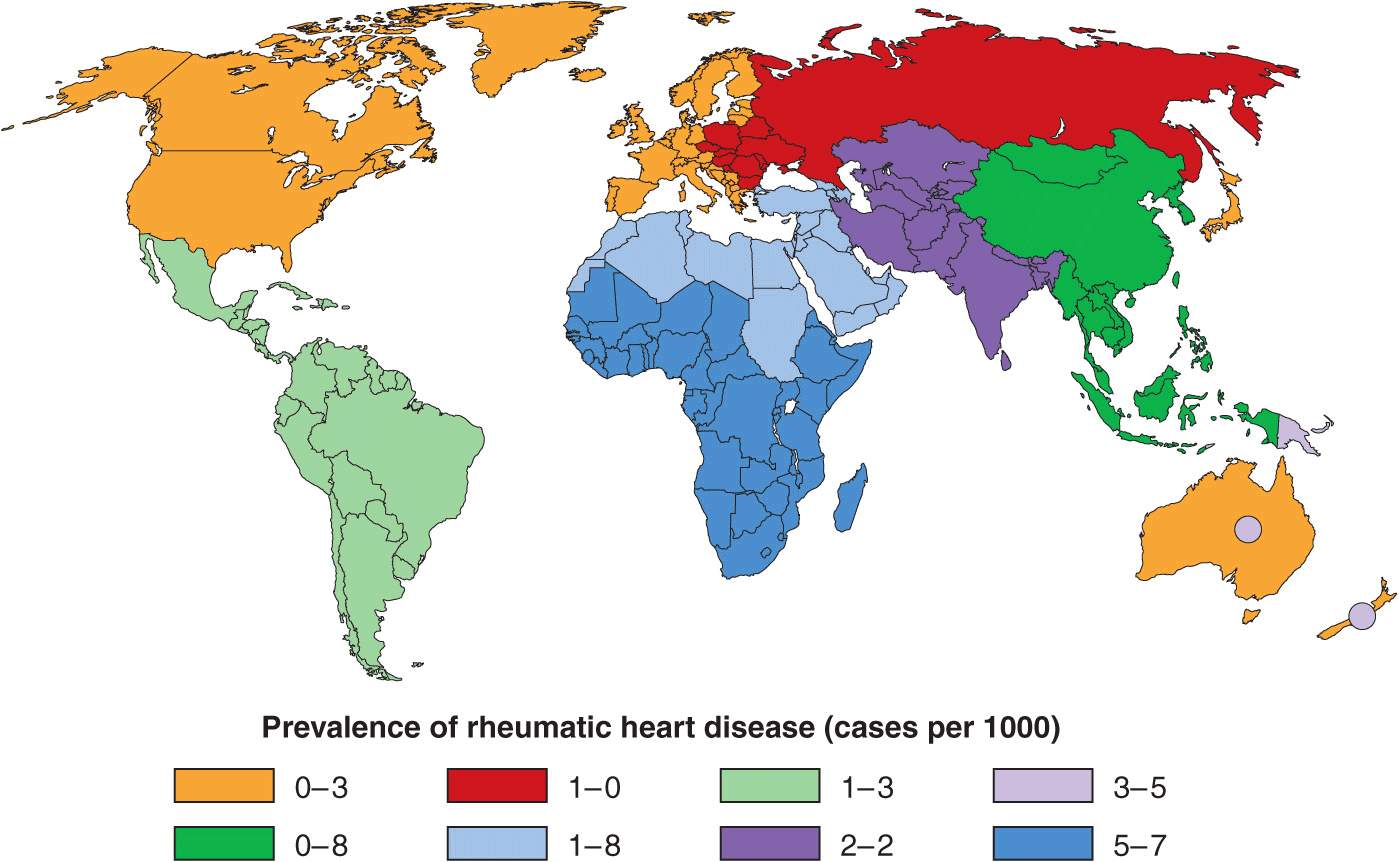
Although ARF and RHD are relatively common in all developing countries, they occur at particularly elevated rates in certain regions. These “hot spots” are sub-Saharan Africa, Pacific nations, Australasia, and the Indian subcontinent (Fig. 26-1). Unfortunately, most developing countries do not currently have coordinated, register-based RHD control programs, which are proven to be cost-effective in reducing the burden of RHD. Enhancing awareness of RHD and mobilizing resources for its control in developing countries is an issue requiring international attention.
FIGURE 26-1
Prevalence of rheumatic heart disease in children aged 5–14 years. Circles within Australia and New Zealand represent indigenous populations, and also Pacific Islanders in New Zealand. (From JR Carapetis et al: Lancet Infect Dis. Copyright 2005; with permission from Elsevier.)
EPIDEMIOLOGY
ARF is mainly a disease of children aged 5–14 years. Initial episodes become less common in older adolescents and young adults and are rare in persons aged >30 years. By contrast, recurrent episodes of ARF remain relatively common in adolescents and young adults. This pattern contrasts with the prevalence of RHD, which peaks between 25 and 40 years. There is no clear gender association for ARF, but RHD more commonly affects females, sometimes up to twice as frequently as males.
PATHOGENESIS
ORGANISM FACTORS
Based on currently available evidence, ARF is exclusively caused by infection of the upper respiratory tract with group A streptococci. Although classically, certain M-serotypes (particularly types 1, 3, 5, 6, 14, 18, 19, 24, 27, and 29) were associated with ARF, in high-incidence regions, it is now thought that any strain of group A streptococcus has the potential to cause ARF. Potential role of skin infection and of groups C and G streptococci are currently being investigated.
HOST FACTORS
Approximately 3–6% of any population may be susceptible to ARF, and this proportion does not vary dramatically between populations. Findings of familial clustering of cases and concordance in monozygotic twins—particularly for chorea—confirm that susceptibility to ARF is an inherited characteristic. Particular human leukocyte antigen (HLA) class II alleles appear to be strongly associated with susceptibility. Associations have also been described with high levels of circulating mannose-binding lectin and polymorphisms of transforming growth factor β1 gene and immunoglobulin genes. High-level expression of a particular alloantigen present on B cells, D8-17, has been found in patients with a history of ARF in many populations, with intermediate-level expression in first-degree family members, suggesting that this may be a marker of inherited susceptibility.
THE IMMUNE RESPONSE
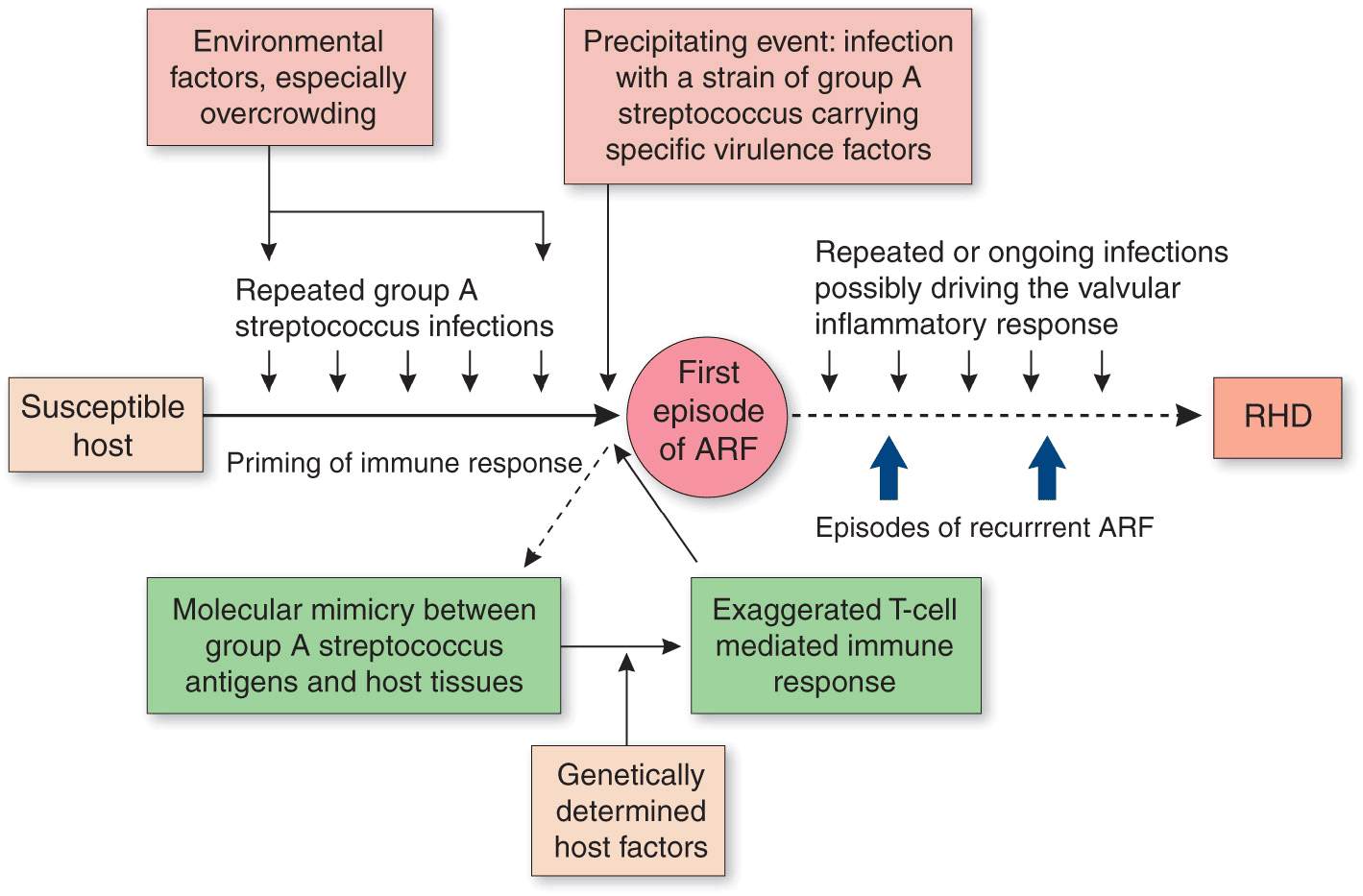
When a susceptible host encounters a group A streptococcus, an autoimmune reaction results, which leads to damage to human tissues as a result of cross-reactivity between epitopes on the organism and the host (Fig. 26-2). Cross-reactive epitopes are present in the streptococcal M protein and the N-acetylglucosamine of group A streptococcal carbohydrate and are immunologically similar to molecules in human myosin, tropomyosin, keratin, actin, laminin, vimentin, and N-acetylglucosamine. It is currently thought that the initial damage is due to cross-reactive antibodies attaching at the cardiac valve endothelium, allowing the entry of primed CD4+ T cells, leading to subsequent T cell-mediated inflammation.
FIGURE 26-2
Pathogenetic pathway for acute rheumatic fever and rheumatic heart disease. (From JR Carapetis et al: Lancet 366:155, 2005. Copyright 2005; with permission from Elsevier.)
CLINICAL FEATURES
There is a latent period of ~3 weeks (1–5 weeks) between the precipitating group A streptococcal infection and the appearance of the clinical features of ARF. The exceptions are chorea and indolent carditis, which may follow prolonged latent periods lasting up to 6 months. Although many patients report a prior sore throat, the preceding group A streptococcal infection is commonly subclinical; in these cases it can only be confirmed using streptococcal antibody testing. The most common clinical presentation of ARF is polyarthritis and fever. Polyarthritis is present in 60–75% of cases and carditis in 50–60%. The prevalence of chorea in ARF varies substantially between populations, ranging from <2% to 30%. Erythema marginatum and subcutaneous nodules are now rare, being found in <5% of cases.
HEART INVOLVEMENT
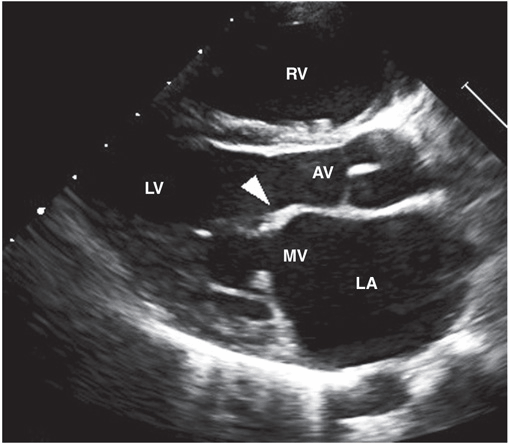
Up to 60% of patients with ARF progress to RHD. The endocardium, pericardium, or myocardium may be affected. Valvular damage is the hallmark of rheumatic carditis. The mitral valve is almost always affected, sometimes together with the aortic valve; isolated aortic valve involvement is rare. Early valvular damage leads to regurgitation. Over ensuing years, usually as a result of recurrent episodes, leaflet thickening, scarring, calcification, and valvular stenosis may develop (Fig. 26-3). Videos 26-1 and 26-2 can be accessed at the following link: http://www.mhprofessional.com/mediacenter/. Therefore the characteristic manifestation of carditis in previously unaffected individuals is mitral regurgitation, sometimes accompanied by aortic regurgitation. myocardial inflammation may affect electrical conduction pathways, leading to P-R interval prolongation (first-degree AV block or rarely higher-level block) and softening of the first heart sound.
FIGURE 26-3
Transthoracic echocardiographic image from a 5-year-old boy with chronic rheumatic heart disease. This diastolic image demonstrates leaflet thickening, restriction of the anterior mitral valve leaflet tip, and doming of the body of the leaflet toward the interventricular septum. This appearance (marked by the arrowhead) is commonly described as a “hockey stick” or an “elbow” deformity. AV, aortic valve; LA, left atrium; LV, left ventricle; MV, mitral valve; RV, right ventricle. (Courtesy of Dr. Bo Remenyi, Department of Paediatric and Congential Cardiac Services, Starship Children’s Hospital, Auckland, New Zealand.)
JOINT INVOLVEMENT
To qualify as a major manifestation, joint involvement in ARF must be arthritic, i.e., objective evidence of inflammation, with hot, swollen, red and/or tender joints, and involvement of more than one joint (i.e., polyarthritis). The typical arthritis is migratory, moving from one joint to another over a period of hours. ARF almost always affects the large joints—most commonly the knees, ankles, hips, and elbows—and is asymmetric. The pain is severe and usually disabling until anti-inflammatory medication is commenced.
Less severe joint involvement is also relatively common but qualifies only as a minor manifestation. Arthralgia without objective joint inflammation usually affects large joints in the same migratory pattern as polyarthritis. In some populations, aseptic mono-arthritis may be a presenting feature of ARF. This may occur because of early commencement of anti-inflammatory medication before the typical migratory pattern is established.
The joint manifestations of ARF are highly responsive to salicylates and other nonsteroidal anti-inflammatory drugs (NSAIDs). Indeed, joint involvement that persists more than 1 or 2 days after starting salicylates is unlikely to be due to ARF. Conversely, if salicylates are commenced early in the illness, before fever and migratory polyarthritis have become manifest, it may be difficult to make a diagnosis of ARF. For this reason, salicylates and other NSAIDs should be withheld—and pain managed with acetaminophen or codeine—until the diagnosis is confirmed.
CHOREA
Sydenham’s chorea commonly occurs in the absence of other manifestations, follows a prolonged latent period after group A streptococcal infection, and is found mainly in females. The choreiform movements affect particularly the head (causing characteristic darting movements of the tongue) and the upper limbs. They may be generalized or restricted to one side of the body (hemi-chorea). The chorea varies in severity. In mild cases it may be evident only on careful examination, while in the most severe cases the affected individuals are unable to perform activities of daily living and are at risk of injuring themselves. Chorea eventually resolves completely, usually within 6 weeks.
SKIN MANIFESTATIONS
The classic rash of ARF is erythema marginatum, which begins as pink macules that clear centrally, leaving a serpiginous, spreading edge. The rash is evanescent, appearing and disappearing before the examiner’s eyes. It occurs usually on the trunk, sometimes on the limbs, but almost never on the face.
Subcutaneous nodules occur as painless, small (0.5– 2 cm), mobile lumps beneath the skin overlying bony prominences, particularly of the hands, feet, elbows, occiput, and occasionally the vertebrae. They are a delayed manifestation, appearing 2–3 weeks after the onset of disease, last for just a few days up to 3 weeks, and are commonly associated with carditis.
OTHER FEATURES
Fever occurs in most cases of ARF, although rarely in cases of pure chorea. Although high-grade fever (≥39°C) is the rule, lower-grade temperature elevations are not uncommon. Elevated acute-phase reactants are also present in most cases. C-reactive protein (CRP) and erythrocyte sedimentation rate (ESR) are often dramatically elevated. Occasionally, the peripheral leukocyte count is mildly elevated.
EVIDENCE OF A PRECEDING GROUP A STREPTOCOCCAL INFECTION
With the exception of chorea and low-grade carditis, both of which may become manifest many months later, evidence of a preceding group A streptococcal infection is essential in making the diagnosis of ARF. As most cases do not have a positive throat swab culture or rapid antigen test, serologic evidence is usually needed. The most common serologic tests are the anti-streptolysin O (ASO) and anti-DNase B (ADB) titers. Where possible, age-specific reference ranges should be determined in a local population of healthy people without a recent group A streptococcal infection.
OTHER POST-STREPTOCOCCAL SYNDROMES THAT MAY BE CONFUSED WITH RHEUMATIC FEVER
Post-streptococcal reactive arthritis (PSRA) is differentiated from ARF on the basis of: (1) small-joint involvement that is often symmetric; (2) a short latent period following streptococcal infection (usually <1 week); (3) occasional causation by nongroup A (μ-hemolytic streptococcal infection; (4) slower responsiveness to salicylates; and (5) the absence of other features of ARF, particularly carditis.
Pediatric autoimmune neuropsychiatric disorders associated with streptococcal infection (PANDAS) is a term that links a range of tic disorders and obsessive-compulsive symptoms with group A streptococcal infections. People with PANDAS are said not to be at risk of carditis, unlike patients with Sydenham’s chorea. The diagnoses of PANDAS and PSRA should rarely be made in populations with a high incidence of ARF.
CONFIRMING THE DIAGNOSIS
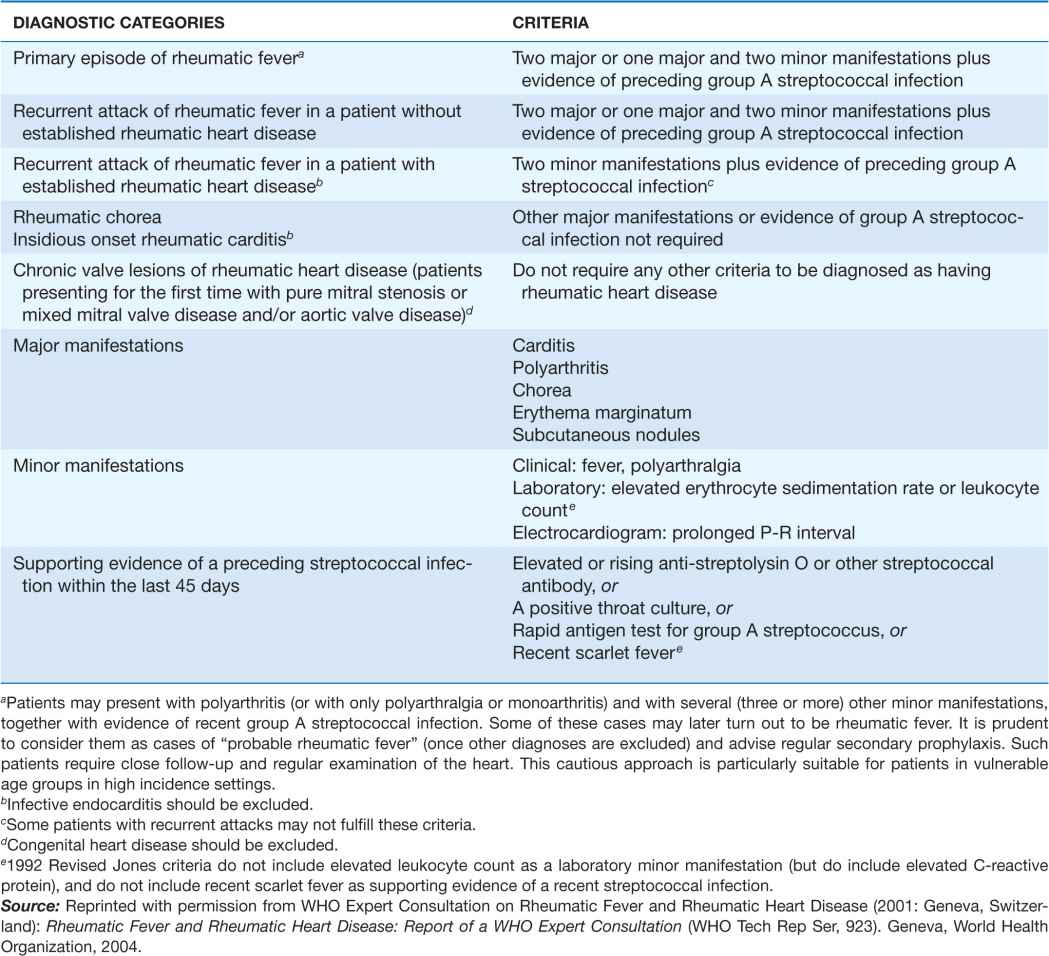
Because there is no definitive test, the diagnosis of ARF relies on the presence of a combination of typical clinical features together with evidence of the precipitating group A streptococcal infection, and the exclusion of other diagnoses. This uncertainty led Dr. T. Duckett Jones in 1944 to develop a set of criteria (subsequently known as the Jones criteria) to aid in the diagnosis. An expert panel convened by the World Health Organization (WHO) clarified the use of the Jones criteria in ARF recurrences (Table 26-1). Because each revision of the Jones criteria since 1944 has reduced sensitivity and increased specificity, in response to the decline in incidence of ARF in high-income countries, there is now concern that they may be too insensitive for countries where ARF incidence remains high. As a result, some countries (e.g., Australia and New Zealand) have developed their own, more sensitive, diagnostic criteria for ARF in their populations (links available at the RHDnet website www.worldheart.org/rhd).
TABLE 26-1
2002–2003 WORLD HEALTH ORGANIZATION CRITERIA FOR THE DIAGNOSIS OF RHEUMATIC FEVER AND RHEUMATIC HEART DISEASE (BASED ON THE 1992 REVISED JONES CRITERIA)
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree