Acute Myocardial Infarction: Early Diagnosis and Management
Eric J. Topol
Frans J. Van De Werf
Overview
The aggressive approach to reperfusion for acute myocardial infarction (MI) continues to be refined. For suitable patients, the cardinal goal is to achieve rapid, complete, and durable restoration of myocardial blood flow. This requires immediate use of either fibrinolytic or catheter-based therapy, and much new data support the latter as the preferred approach when this can be made available. The admission electrocardiogram (ECG), which provides the location and approximate size of the infarct, is remarkably important for prognosis, and combined with the main clinical parameters of age (the single most important parameter), heart rate, blood pressure, and Killip class, more than 90% of prognostic information is quickly assembled.
Patients without ST-segment elevation have non–Q-wave MI or unstable angina and are generally treated the same way, except without the use of fibrinolytic agents. Therapy with aspirin, anticoagulation, and β-blockade is appropriate in most patients. If primary coronary intervention is not used, consideration for coronary angiography, either on an urgent or elective basis, and coronary revascularization, are key decisions that need to be made as a function of the patient’s risk profile, the occurrence of recurrent ischemia, and resource availability.
Although there has been considerable progress in recent years, our current armamentarium leaves many patients with suboptimal reperfusion at the tissue level. This problem has not yet been adequately addressed despite attempts with platelet glycoprotein IIb/IIIa inhibitors and other antiplatelet agents, better anticoagulants, antiinflammatory agents, and emboli capture devices for catheter-based reperfusion. Undoubtedly,
we need to more effective restoration of epicardial and tissue-level perfusion.
we need to more effective restoration of epicardial and tissue-level perfusion.
Glossary
Myocardial reperfusion
Restoration of coronary blood flow through the infarct-related artery.
Primary angioplasty
Use of balloon angioplasty to achieve myocardial reperfusion.
Rescue angioplasty
For patients who fail fibrinolytic therapy, the use of catheter-based reperfusion as a fallback approach in the early hours of the event.
Facilitated reperfusion
The use of a pharmacologic approach as a bridge to catheter-based reperfusion.
TIMI-3 flow
Brisk, complete flow as assessed by the Thrombolysis in Myocardial Infarction semiquantitative scoring system.
Pathophysiology
Coronary atherosclerotic disease is the underlying substrate in nearly all patients with acute MI. The initiating event is a crack or fissure in the diseased arterial wall, which occurs as a result of loss of integrity of the plaque cap (the fibrous tissue overlying the plaque and partitioning the atheroma from the arterial lumen). The fissure or even frank plaque rupture leads to exposure of subendothelial matrix elements such as collagen, stimulating platelet activation and thrombus formation. Furthermore, tissue factor is released with the arterial injury, which directly activates the extrinsic coagulation cascade and promotes the formation of fibrin. If an occlusive thrombus forms, patients may develop an acute ST-segment elevation MI unless the subtended myocardium is richly collateralized. On the other hand, the thrombus formed may not be occlusive, but rather mural, and the patient may develop unstable angina or non–ST-segment elevation changes on the ECG (ST-depression or T-wave changes), which denote the lack of a “current of injury” or full-thickness (subendocardial to epicardial) myocardial ischemia.
Understanding the reasons why plaques crack may provide a better means of preventing acute MI, rather than intervening at the late phase after the event has been initiated. Plaques that rupture or fissure tend to have a thin fibrous cap, a high lipid content, few smooth muscle cells, and a high proportion of macrophages and monocytes (1). These mononuclear cells are conceived as a major trigger in plaque rupture by their release of such proteases as monocyte chemotactic protein and matrix metalloproteinases (e.g., collagenases, stromelysin, elastases), which chemically digest the plaque cap. Of note, the 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase inhibitors have been shown to reduce the incidence of MI, and this is likely related to reduction in lipid content, as well as a favorable antiinflammatory effect on the cellular plaque constituents and chemokines (2). Loss of integrity of the arterial wall and platelet thrombus, with cessation of coronary blood flow through the infarct-related artery, thus drives myocardial ischemia and injury. As elegantly described by Reimer and Jennings, the wavefront of necrosis extends from the subendocardium to the subepicardium (3), and the extent of necrosis varies as a function of collateral flow, the length of time that coronary blood flow has halted, and the extent of diminution of coronary blood flow. In many patients, there is a stuttering quality of MI with severe pain often denoting the cutoff of blood supply, and less chest pain with partial, albeit insufficient, reflow. This dynamic quality of the infarct vessel blood flow pattern in acute MI (altered vasomotor tone or spasm) is likely related to the release of vasoactive amines from the activated platelets and loss of endothelial function.
The thrombus that occludes the coronary artery is a mixture of white (platelet-rich) and red (fibrin- and erythrocyte-rich) clots. In some patients, there is a more dominant role of platelets, whereas in others predominantly fibrin-rich thrombus at the arterial injury site is found. Stagnation thrombosis results from the lack of blood flow through the infarct vessel, leading to calumniation of red thrombus proximal to the original occlusion site (4). There is rarely frank herniation of the plaque occluding the lumen, which is known as plaque disaster (4).
On the other hand, the mural thrombus in patients without ST-segment elevation MI is apt to be platelet rich and not accompanied by stagnation; there is not sustained cutoff of coronary blood flow. Depending on the extent and duration of ischemia, the patient may not experience any myocardial necrosis (unstable angina) or develop myocardial damage (non–ST-elevation or non–Q-wave infarction). Beyond what is occurring at the arterial injury site and proximal to it, there is the potential for embolization of atheroma constituents or platelet thrombus distally. This is not typically found at routine postmortem but requires careful histologic inspection (5). When this occurs, a further explanation to cessation of myocardial blood flow is provided.
Incidence and Significance
The true incidence of acute MI is unknown. Beyond the significant proportion of patients who die before reaching the hospital, estimated at 300,000 to 400,000 patients annually in the United States (6), it is estimated that approximately 1 million patients present to a hospital each year with some type of MI as the principal diagnosis (7). Of these, we know that roughly 200,000 patients receive reperfusion therapy in the United States per year, and that this represents only 20% to 30% of the patients who are assessed for eligibility for aggressive management (8,9). The breakdown of infarctions is also unclear, with a split between the classic ST-segment elevation and the non–ST-segment elevation. The latter group is increasingly common but difficult to differentiate from unstable angina on presentation. It is estimated that the majority of patients with acute coronary syndromes now present with no definitive ECG changes, or have abnormal ST-segment depression or T-wave inversion.
The incidence and mortality resulting from MI is on the decline. Not only has the fatality rate been reduced as better therapies have evolved, but the absolute number of MI events has continued to decrease since the 1970s. This is likely attributable to the many advances in preventive cardiology, including treatment of hypertension, avoidance of smoking, management of hypercholesterolemia, improved diet and exercise, and the prophylactic use of aspirin. It may also be an outgrowth of the high rates of surgical and percutaneous coronary revascularization in the United States, where the treatment of angina is more apt to be bypass surgery or stenting, rather than medical management. Although prevention of MI is not a prominent effect of coronary revascularization, intervening early in the course of atherosclerotic coronary disease may change its long-term natural history. A pronounced effect on reducing the incidence of MI appears to have resulted from the use of HMG-CoA reductase inhibitors (2), which has led to the concept that acute MI may even be “extinct” someday (10). Clearly, the most important initiatives for the condition of MI are in its prevention, because once the process of plaque rupture is unleashed, it is much more difficult to interrupt.
Although the incidence is declining, acute MI remains the principal cause of death in Western society and it is projected by 2020 for this to be the case worldwide (11). With the many rapid advances that have occurred in this field, the serious and potentially catastrophic outcome of an MI event has been underestimated in more recent years. Although reperfusion has made important progress in lowering mortality, most patients with acute MI are not eligible for this therapy and face in-hospital death rates of 10% to 20% (8). With the increasing proportion of our population being represented by the elderly, who have a high incidence of fatality even with reperfusion therapy, MI remains as the most critical single event in medicine.
Diagnosis
Acute MI is a clinical syndrome for which a constellation of subjective and objective parameters need to be assessed. The diagnosis must be obtained rapidly and accurately, and misdiagnosis can have catastrophic sequelae. The individual components of making the diagnosis are discussed separately, but it is their integration that facilitates the accuracy and speed of the clinical syndrome recognition.
History
The classic symptoms of MI are intense, oppressive, durable, excruciating chest pressure, with an impending sense of doom and radiation of the pain to the left arm. However, the other symptoms of chest heaviness or burning, radiation to the jaw, neck, shoulder, back, or both arms may be encountered. Indigestion is common, especially with inferior wall MI. Nausea (particularly) and vomiting are typical. Profuse diaphoresis is also a frequent characteristic. Taken together, the patient with a clear-cut presentation is experiencing a unique, discrete, painful event that has induced fear. However, the subtleties of the history are more common and challenging. It is important to ask whether there were premonitory signs of chest discomfort (not necessarily pain) in the preceding week or two. Pain or discomfort may be completely localized to the arm or shoulder. Quite commonly, only the symptoms of indigestion and nausea prevail, such that the patient attributes the episode to heartburn and resorts to taking antacids.
The identification of risk factors, such as smoking, known cholesterol elevation, diabetes, hypertension, and family history, is a supportive piece that helps to put the acute history into context. The chest discomfort that causes the patient to seek medical attention is usually sustained (>20 minutes), but can be stuttering.
Other accompanying symptoms include dyspnea, which is of concern because it may denote incipient congestive heart failure or, alternatively, is an outgrowth of the patient’s anxiety. Palpitations or syncope are unusual, but a history of lightheadedness or dizziness and presyncope often reflects the underlying vagotonia or bradyarrhythmias. When syncope, or an out-of-hospital arrest, has occurred there is a high likelihood of ventricular tachycardia as an explanation.
The differential diagnosis is quite broad (because many conditions can masquerade as acute MI) including aortic dissection, pericarditis, esophagitis, myocarditis, pneumonia, cholecystitis, and pancreatitis. Of these conditions, it is always worth considering that the patient has aortic dissection until proven otherwise, so that this diagnosis is not missed. Although considerably less common than acute MI, the therapies for the two conditions are entirely different and, for example, the use of fibrinolytic therapy for aortic dissection could be disastrous.
Physical Examination
The patient appears to be in distress and may even be writhing in pain. Pallor is common. The pulse is usually regular, although ventricular extrasystoles may be present. Bradycardia or tachycardia is helpful in understanding the infarct location, the effect on the conduction system, the vagal tone, and the extent of myocardium at jeopardy. Significant tachycardia (pulse >120) is worrisome and usually denotes an extensive MI, although a “hyperdynamic” subset of patients who have relatively small infarcts, but are hyperadrenergic, may be encountered. The blood pressure is typically elevated owing to the body’s response to pain. Hypotension is either due to vagotonia, dehydration, a right ventricular infarction, or impending power failure.
Major examination findings to be aware of include whether there is elevation of jugular venous pressure, the character and location of the apical impulse, the splitting of the second heart sound, the presence of a third or fourth heart sound, a mitral regurgitant murmur, and whether there are rales. Examination of the peripheral pulses and the extremities is important. Collectively, this information provides a sense of the size of the myocardial infarct. If a third heart sound is present, along with rales halfway up the posterior chest fields (Killip class II), a large anterior wall MI is likely present. On the other hand, a normal examination suggests either a small infarction or that more extensive myocardial damage has not yet occurred.
Electrocardiography
It is imperative to obtain a 12-lead ECG as quickly as possible to secure the diagnosis. The presence of a true normal ECG rules out the occlusion of a major epicardial vessel at the moment the tracing was obtained. Hyperacute, tall T-wave changes are the first manifestations of acute coronary occlusion, but are frequently not present when the patient reaches the hospital for medical attention. The presence of ST-segment elevation is the principal feature that denotes “current of injury” and should be associated with reciprocal depression in contralateral leads. If only minimal (1–2 mm) ST elevation is present, then either the patient has collaterals to the infarct territory, the vessel is not fully occluded, or there has already been evolution of the ECG changes. If only ST-segment depression or T-wave inversion or both are manifest, this may denote either unstable angina or a non–ST-elevation (non–Q-wave) MI. This usually is not associated with an occluded infarct vessel, but rather one that is stenotic with myocardial ischemia. If a patient has a normal ECG, but the history is suggestive or even compelling, it is vital to observe the patient over an extended period (6–24 hours) to obtain additional ECG tracings and to determine if the chest discomfort or other symptoms recur. Certainly transient but marked ischemia can resolve before the patient has an ECG and a normal ECG can be recorded. It is worthwhile to give sublingual nitroglycerin (0.4 mg) to a patient with marked ST-segment elevation to see if this represents coronary artery spasm while more definitive therapy (vide infra) is being initiated. If the patient’s chest pain and ECG quickly revert to normal after nitroglycerin, this strongly suggests vasospasm as the principal trigger.
In patients with ST-segment elevation largely confined to the right precordial leads (V1–V2), it is important to differentiate ST-segment elevation owing to current of injury and fast (early) repolarization, which is a normal variant and especially common in young African-American men. Early repolarization is diminished or undetectable when the heart rate is increased, so that if the ECG remains equivocal it may be helpful to have
the patient do some sit-ups to increase the heart rate, and then repeat the ECG.
the patient do some sit-ups to increase the heart rate, and then repeat the ECG.
Creatine Phosphokinase
Plasma levels of creatine phosphokinase (CK), or of the isoenzyme CK-MB, which is primarily localized in the heart, are unhelpful in making the initial diagnosis. It takes at least 6 hours for there to be an enzyme “leak,” which denotes myocardial cell necrosis. Enzymes should be assessed every 8 hours for the first 24 hours, and longer if the peak is not firmly established. Peak CPK occurs earlier when there has been successful reperfusion (12,13). This enzyme is much more helpful in gauging the size of the MI than in making the diagnosis.
Troponin T and I
More sensitive measures of myocardial necrosis have become available, which have a similar or even greater obligatory lag in appearing in the blood (as CK or CK-MB) but appear to detect cell damage more readily (14). The troponins are part of the tropomyosin-binding protein of the contractile apparatus of cardiac myocytes and therefore are highly specific for cardiac origin. A rapid bedside assay for troponin is available and is a practical, rapid way to assess patients with ischemic symptoms and non–ST-segment elevation. The off-line quantitative assays of both troponin T and I have been particularly helpful in differentiating risk, better than CK, in patients with unstable angina and non–ST-elevation MI. Both offer a more sensitive means of not only diagnosing whether infarction has occurred, but also discriminating the risk. However, the real utility of tests such as troponin goes beyond the determination of risk level to favorably alter the prognosis of the patient by knowledge of whether the test result is abnormal or not. Indeed, responses of patients to either IIb/IIIa inhibitors or low-molecular-weight heparins are predicted by abnormal troponin. These sensitive markers should be applied routinely for patients without ST-segment elevation. Troponins offer little incremental value in classic ST-elevation MI.
Echocardiography
The diagnosis of acute MI cannot be made by echocardiography because diagnosis is based on the combination of symptoms, the ECG findings, and the enzyme abnormalities. However, there are several findings that may be considered ancillary from a two-dimensional echocardiography, including a segmental wall abnormality and hyperkinesis of the contralateral wall. If a segment is akinetic, dyskinetic, or severely hypokinetic, it is not possible to know whether there was ischemia, with attendant dysfunction or stunning, or irrevocable damage due to necrosis. This can only be differentiated by serial echocardiographic examination. However, the finding of lack of hyperkinesis of the contralateral territory (e.g., the anterolateral wall in an inferior MI) in the acute setting suggests that the infarct vessel has recanalized or that there is multivessel coronary disease. This finding may be especially valuable in assessing a patient with congestive heart failure or cardiogenic shock; the main compensation of the ventricle to preserve its global ejection fraction is preempted if there is a significant stenosis of a major epicardial vessel supplying the noninfarcted territory. In patients with inferior MI, an echocardiogram of the right ventricle is helpful in demonstrating dilation or hypocontractility and is more sensitive than the right precordial ECG lead ST-segment elevation. A significant prior MI is also diagnosed by scarring and thinning of a specific territory and can easily be differentiated from an acute MI by its echocardiographic appearance.
Angiography
On occasion, even with all of the tools outlined, the diagnosis is uncertain. This may be the result of atypical symptoms and an ECG that is difficult to interpret. In a patient in whom reperfusion therapy is contemplated, an approach that can rapidly establish the diagnosis is emergency coronary angiography. By demonstrating an acutely occluded infarct vessel, with the characteristic appearance of thrombus or a cutoff sign at the point of occlusion, coupled with left ventriculography to ascertain the segmental wall motion profile, angiography can at times be helpful in a difficult diagnosis. Examples of patients who may present with ambiguity are those with an acute myocarditis with diffuse ECG changes, or patients with prior bypass surgery and previous MI, or those without a characteristic pattern on ECG. Furthermore, angiography can serve as the foundation for primary angioplasty to achieve reperfusion.
Major Subgroups at Presentation
By Electrocardiography
In Table 19.1, the five types of ST-segment elevation MI are presented. These represent specific patterns of ECG abnormalities that correlate with a clinical presentation, the underlying coronary anatomy, and prognosis. The most worrisome type is the proximal left anterior descending (LAD) MI, often referred to as the widow-maker infarction, which carries a high mortality and is attributed to an occlusion of the LAD before or at the first septal perforator. All of the precordial leads and I and aVL show ST-segment elevation. The proximal location of occlusion is associated with compromised perfusion to the His-Purkinje conduction tissue owing to loss of septal supply and often accompanied by a new bundle branch block. Usually, left anterior hemiblock or right bundle branch block is present, but bifascicular blocks, left bundle branch block, or Mobitz II atrioventricular block are all possible. Cardiogenic shock or power failure is not unexpected in this subgroup, unless there has been effective reperfusion established.
In contrast, occlusion of the LAD just distal to the first septal perforator is an anterior MI, which is less serious and called the mid-LAD infarction. Although the ECG may be indistinguishable from that of the proximal anterior MI patient with respect to the leads with ST elevation, there is no conduction disturbance. Cardiogenic shock in these patients is considerably less frequent, as restriction of the damage to the anterolateral and anteroapical segments spares the proximal interventricular septum. If shock is present, one should be concerned about prior myocardial damage or other noncardiac causes such as massive hemorrhage. Heart failure can occur, and the complications of ventricular aneurysm with potential of apical thrombus are common, especially if reperfusion has been delayed or is unsuccessful.
One segment distal in the LAD, sparing a large diagonal, represents the distal LAD infarction, which is less common. Only leads V1 to V4 are affected, but still there is the potential for apical hypokinesis, thrombus formation, and a milder form of the mid-LAD infarct clinical syndrome. Importantly, cardiogenic shock cannot result from this type of infarction per se.
Two types of infarcts that are relatively small are due to less significant territory affected. These are the LAD diagonal branch “lateral” MI, involving only leads I, aVL, V5, and V6,
also classified in the distal LAD territory MI, and the small inferior MI, with ECG ST elevation confined to leads II, III, and aVF. The latter is usually attributed to a distal right coronary artery lesion or branch (posterolateral or posterior descending), but in patients with a dominant left circumflex, it may be a branch from this vessel. Both of these types of MIs are usually uncomplicated and rarely associated with serious outcomes such as congestive heart failure or significant arrhythmias.
also classified in the distal LAD territory MI, and the small inferior MI, with ECG ST elevation confined to leads II, III, and aVF. The latter is usually attributed to a distal right coronary artery lesion or branch (posterolateral or posterior descending), but in patients with a dominant left circumflex, it may be a branch from this vessel. Both of these types of MIs are usually uncomplicated and rarely associated with serious outcomes such as congestive heart failure or significant arrhythmias.
TABLE 19.1 A Classification of Acute MI Based on Electrocardiographic Entry Criteria with Angiographic Correlation | |||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |||||||||||||||||||||||||||||||||||
Patients with moderate or large inferior MIs are a key subgroup, which is heterogeneous, representing a spectrum of involvement of the inferior, posterior, lateral, and right ventricular myocardial involvement. The proximal, dominant right coronary artery is responsible for supplying all of these territories and can result in a large and potentially catastrophic event. The ECG leads involved include II, III, and aVF, and additional changes in the V5, V6 lateral leads, right ventricular leads (V1 or V3R, V4R), or posterior leads (R/S ratio >1 in V1, V2, with or without ST depression, ST elevation in aVR). The largest inferior MI involves the composite of all of these territories. Dreaded complications, such as power failure or cardiogenic shock due to a large right ventricular infarct, or the development of a ventricular septal defect due to extensive distal septal necrosis, are possible. In all patients with inferior MI, a systematic approach to obtaining the right precordial ECG must be incorporated. Although right ventricular lead ST elevation of 1 to 2 mm in V3R or V4R is highly specific for right ventricular infarct, the sensitivity is suboptimal, and one should carefully examine the patient for elevated jugular venous pressure, a right-sided S3 gallop, and, in cases in which there is uncertainty, perform either or both echocardiography or right-sided heart catheterization. Inferior MI is characterized by hypervagotonia, with bradycardia and hypotension that is responsive to atropine (0.6–1.0 mg intravenously). Type I atrioventricular block (Wenckebach) is exceedingly common with inferior MI, particularly if continuous ECG monitoring is reviewed. The conduction disturbances and bradyarrhythmias of inferior MI are usually benign, most often not requiring specific therapy other than occasional atropine.
Diagnosis of a true posterior infarction is rare, but the posterior wall is the least well expressed on the 12-lead ECG, such that occlusion of the left circumflex branches, which are usually responsible for the true posterior myocardial territory, can be missed. When the findings of ST depression in V1 to V4 are present, especially when accompanied by an R greater than S wave in V1 or V2 (or both), the ECG is highly supportive of a posterior MI. The ST depression follows the “mirror rule” as the reflection of the tracing would fit the conventional ST-elevation pattern that one would expect in other MI locations. Posterior wall MIs are usually well tolerated, but it is imperative to interrogate the possibility of concomitant involvement of the right ventricle and free wall of the left ventricle.
All of the patterns described have associated reciprocal ECG changes, which are important to confirm the diagnosis. For example, most patients with inferior MI have ST depression in either leads I, aVL, or precordial leads V1 to V4, and the prognostic significance of this latter finding has been debated for many years. The extent of reciprocal changes is usually similar to the primary current of injury, such that if there is 5 mm of ST elevation, there is more pronounced evidence of contralateral, reciprocal ECG changes. For the most part, this finding is useful for confirming the diagnosis and represents an ECG contrecoup expression, which does not carry vital clinical prognostic information. In most series, however, there is some incremental risk of adverse outcomes as a function of the extent and severity of reciprocal ST-segment depression (15).
Repeat 12-lead ECGs are vital 60 to 90 minutes after administration of fibrinolytic therapy to detect whether tissue-level reperfusion has occurred. As shown in Table 19.2, with the early repeat ECG in many large-scale trials (16,17,18,19,20,21) the prognosis of patients is greatly differentiated by whether there has been greater than or equal to 70% resolution of ST-segment elevation. This has come to serve as the “lie detector” or “truth serum” as an inexpensive, readily available means of assessing
tissue-level reperfusion. It is also worthwhile to recheck the ECG after catheter-based reperfusion because approximately 20% to 30% of patients do not achieve ST-segment resolution, even in the presence of TIMI flow grade 3, and carry an unfavorable prognosis (22). This criterion of incomplete ST-segment resolution has served as the basis for rescue catheter-based reperfusion trials, the results of which will be reviewed.
tissue-level reperfusion. It is also worthwhile to recheck the ECG after catheter-based reperfusion because approximately 20% to 30% of patients do not achieve ST-segment resolution, even in the presence of TIMI flow grade 3, and carry an unfavorable prognosis (22). This criterion of incomplete ST-segment resolution has served as the basis for rescue catheter-based reperfusion trials, the results of which will be reviewed.
Non–ST-Segment Elevation
Patients with acute ECG changes but without ST-segment elevation fit into the non–ST-elevation category. This was formerly known as subendocardial, non–Q-wave MI, but the reperfusion era has provided ample evidence that many patients with initial ST elevation do not go on to evolve a Q-wave MI (23). Giant T-wave inversion, characteristic of a proximal LAD lesion if occurring throughout the precordial leads, is an example of a non–ST-elevation MI with a discrete ECG pattern. However, many of the ECGs in this patient group do not fit a particular pattern of myocardial necrosis or ischemia, and at the time of evaluation, one is uncertain whether the changes are fixed or transient. Clearly, ST-segment depression is more ominous than T-wave inversion for 30-day mortality or nonfatal reinfarction. In a large-scale trial, the death rates were 6.8% versus 1.4%, and the composite event rates were 12.4% and 6.8%, respectively (24). Especially worrisome is the finding of global ischemia, when there is an ST depression in virtually every lead except aVR (where there is elevation); this frequently denotes a left main or equivalent non–ST-elevation MI.
By Hemodynamic (Killip) Class
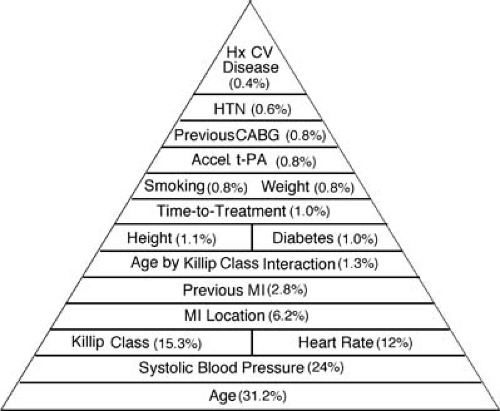
Beside the ECG, which establishes the location of the infarct and often the precise location of the affected coronary artery, it is important to establish the patient’s hemodynamic class. The Killip categories are most useful and validated for this purpose (25). Killip class I is the most common presenting class, occurring in 85% of patients with MI, and, by definition, with no evidence of heart failure. Early heart failure, as manifested by bibasilar rales and at times an S3 gallop, is present in Killip II, the category that approximately 10% of patients present with. Pulmonary edema, as denoted by Killip III, and cardiogenic shock, classified as Killip IV, are uncommon, collectively accounting for 5% of patients in large-scale trials. In evaluating the patient’s risk, five simple baseline parameters have been demonstrated, explaining more than 90% of the prognostic information for 30-day mortality. As shown in the pyramid (Fig. 19.1), the key five characteristics are age, systolic blood pressure, Killip class, heart rate, and location of the MI (26). Thus, evaluation of the patient’s age, ECG, and hemodynamics provides crucial information that stratifies risk and may be helpful in guiding therapy. More recently, the prognostic value of the baseline serum creatinine has been highlighted (27).
Patient Selection for Reperfusion Therapy
All patients with ST-segment elevation MI who present within 12 hours from the onset of symptoms should be considered for myocardial reperfusion therapy. The only definite contraindications for fibrinolytic therapy are any previous intracranial hemorrhage, active bleeding, and recent stroke, trauma, or major surgery. Relative contraindications include severe or uncontrolled hypertension (systolic blood pressure >180/110 mm Hg), any previous cerebrovascular history, prior gastrointestinal hemorrhage, active menstruation, pregnancy, prolonged cardiopulmonary resuscitation (>10 minutes), noncompressible vascular punctures, and coumadin therapy with an international normalized ratio of greater than 2. For patients with relative contraindications to fibrinolytic therapy, primary angioplasty is the preferred reperfusion therapy.
Patients with new bundle branch block are also considered suitable candidates for reperfusion on the basis of the collaborative overview of the large-scale controlled trials of fibrinolytic therapy (28). Furthermore, right bundle branch block does not obscure ST elevation, and ST-segment elevation MI can frequently be diagnosed in the presence of left bundle branch block (29). Thus, for the most part, ST-segment elevation is the hallmark ECG feature guiding the use of reperfusion therapy along with symptoms, appropriate timing, and consideration of the clinical profile.
The benefit of reperfusion therapy generally appears to be independent of age, gender, and most baseline characteristics. However, the patients who derive the most benefit are patients
treated earliest, those with anterior MI, and, in general, those with the highest risk. The critical dogma is to restore myocardial perfusion as quickly as possible. Accordingly, in a hospital with an experienced team for acute MI catheter-based intervention with an open laboratory, there is no question that percutaneous coronary intervention (PCI) would be the optimal strategy. The field of patients eligible for reperfusion is extended with the use of PCI, because of the contraindications to fibrinolytic therapy (30). Furthermore, owing to the relative lack of efficacy of fibrinolytic intervention in specific settings, such as in patients with cardiogenic shock and those with prior bypass surgery, PCI is preferred. As reviewed here, whenever available on a timely basis, even if this necessitates interhospital transfer, PCI should now be considered the reperfusion strategy of choice.
treated earliest, those with anterior MI, and, in general, those with the highest risk. The critical dogma is to restore myocardial perfusion as quickly as possible. Accordingly, in a hospital with an experienced team for acute MI catheter-based intervention with an open laboratory, there is no question that percutaneous coronary intervention (PCI) would be the optimal strategy. The field of patients eligible for reperfusion is extended with the use of PCI, because of the contraindications to fibrinolytic therapy (30). Furthermore, owing to the relative lack of efficacy of fibrinolytic intervention in specific settings, such as in patients with cardiogenic shock and those with prior bypass surgery, PCI is preferred. As reviewed here, whenever available on a timely basis, even if this necessitates interhospital transfer, PCI should now be considered the reperfusion strategy of choice.
Treatment
Fibrinolytics or Catheter-Based Reperfusion
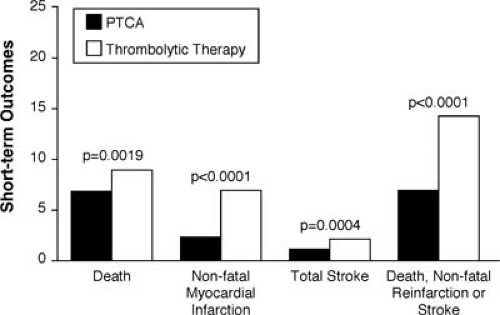
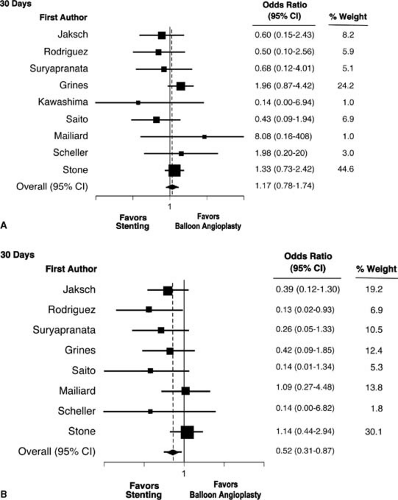
For many years there has been an active debate as to which reperfusion therapy is preferred—fibrinolytics or catheter-based reperfusion. Cumulatively, 23 randomized trials in 7,739 patients, show an advantage for primary angioplasty (31). The advantages, shown in Figure 19.2, are manifest in short-term reduction in mortality, reinfarction, and stroke. The vast majority of patients in these 23 trials underwent balloon angioplasty, but in recent years the use of stenting has largely replaced balloon angioplasty for catheter-based reperfusion. This change in practice was solidified with the results of randomized trials comparing balloon angioplasty with stenting for acute MI (31,32,33,34,35,36,37,38,39,40,41). As summarized in pooled analysis of the nine trials, primary stenting offered significant advantages compared with balloon angioplasty for the reduction of reinfarction (Fig. 19.3) and repeat target vessel revascularization. However, in this combined study of 4,433 patients, there was actually a 17%, increase in mortality at 30 days, albeit not statistically significant, for stenting compared with balloon angioplasty (31).
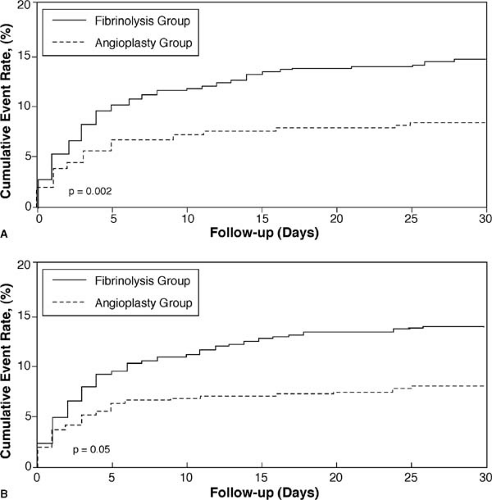
The advantages of catheter-based reperfusion over intravenous fibrinolytics was greatly extended by randomized trials that incorporated transfer of patients to a facility in which the PCI could be performed. The largest trial, the Danish Multicenter Randomized Study on Fibrinolytic Therapy Versus Acute Coronary Angioplasty in Acute Myocardial Infarction (DANAMI-2), showed a striking advantage for reduction of the composite of death, disabling stroke, or reinfarction, both for referral hospitals (requiring interhospital transfer) and invasive treatment centers (Fig. 19.4) (42). In this trial, the catheter-based reperfusion consisted of stents in 93% of the patients.
Other trials comparing fibrinolytics and interhospital transfer for catheter-based reperfusion have confirmed and extended the DANAMI-2 results (43,44,45). Interestingly, for the three trials summarized in Figure 19.5, there is remarkable similarity of the event rates among the transported patients, and the magnitude of reduction of events of death, reinfarction, and stroke (43). In the CAPTIM trial, prehospital administration of a lytic agent followed by rescue PCI, if needed, was associated with a lower mortality when compared with primary PCI, especially when the lytic was given very early(<2 hours) after onset of symptoms (46). Similar results were found in registries in Europe. These results should be viewed in the light of the negative outcomes after facilitated PCI with lytic agents (see below).
Analgesia and Supportive Measures
The first step in treating the patient, while more definitive therapy is being prepared (such as fibrinolytics or transfer to a cardiac catheterization laboratory), is to make the patient comfortable via supplemental oxygen (usually nasal cannula at 2 L/min) and morphine (2–4 mg intravenously and repeat as necessary). Before using morphine, it is helpful to have quickly tried sublingual nitroglycerin to determine whether there is a reversible component of the ischemia, pain, and ECG changes.
Aspirin
The use of aspirin is a cornerstone of therapy for patients with acute coronary syndromes. It should be initiated as quickly as possible when the diagnosis is made, at a dose of 160 mg by chewable administration (two 80-mg “baby” aspirin) or a 325-mg orally administered tablet. No enteric coated formulation of aspirin should be used. The validation for the importance of aspirin in this setting is derived from the landmark International Studies of Infarct Survival (ISIS-2) trial (Fig. 19.6), which showed it is lifesaving (47). Since this trial, aspirin has been used in virtually all patients with acute MI, and other trials have provided strong evidence for its use in unstable angina and non–ST-elevation MI. The dose that has been routinely recommended is 325 mg/day. For long-term use, a daily dose of 75 to 81 mg may represent the best balance of efficacy and safety, but no definitive randomized trials of aspirin dosing are available.
Clopidogrel
Recent trials have addressed the addition of clopidogrel to aspirin, as compared to aspirin alone, in the setting of ST-segment elevation MI. In the CLARITY trial of 3,491 patients (48) with a hybrid angiographic and clinical composite endpoint (Fig. 19.7A), there was a 36% reduction of an occluded infarct artery at early angiography, death, or recurrent MI. This trial focused on patients younger than 76 years, such that the potential for bleeding complications, chiefly occurring in the elderly when angiography is performed early, was not directly assessed. In the COMMIT trial conducted in China with 45,852 patients (49), there was a 9%, statistically significant reduction
of death, reinfarction, or stroke with the addition of clopidogrel on top of aspirin (Fig. 19.7B). These two trials taken together suggest strong consideration should be given that in patients for whom reperfusion therapy is appropriate, that early clopidogrel is added to aspirin. A loading dose of 300 to 600 mg may be optimal, but no loading was used in COMMIT and likely an insufficient loading (300 mg) was used in CLARITY. The desired rapid antiplatelet action would support the higher loading dose for this orally administered medicine.
of death, reinfarction, or stroke with the addition of clopidogrel on top of aspirin (Fig. 19.7B). These two trials taken together suggest strong consideration should be given that in patients for whom reperfusion therapy is appropriate, that early clopidogrel is added to aspirin. A loading dose of 300 to 600 mg may be optimal, but no loading was used in COMMIT and likely an insufficient loading (300 mg) was used in CLARITY. The desired rapid antiplatelet action would support the higher loading dose for this orally administered medicine.
Time to Treatment
The time to treatment is a pivotal parameter in reperfusion. Patients treated in the first hour have the highest absolute and relative mortality benefit (50). This observation has led to the first 60 minutes to be referred to as the golden hour of reperfusion. The dominant explanation for the exaggerated benefit appears to be related to prevention of myocardial damage, as thallium studies have indicated the ability to prevent an MI in up to 40% of such patients (51). However, as judged from the high placebo group mortality of patients treated in the first hour, it may be that patients who present early (e.g., within 30 minutes of onset of symptoms) have a larger MI and are therefore preselected to derive pronounced benefit. Certainly all of the trials convey an inverse relationship between treatment onset and survival benefit with little to no beneficial effect for patients treated at 12 hours or beyond. Two large-scale trials have been dedicated to the issue of late therapy (52,53), and both suggest that treatment benefit is restricted to the first 12 hours. The benefit of prehospital initiation of fibrinolysis (17% reduction in hospital mortality when compared with in-hospital initiation in a metaanalysis by Morrison et al. [54]) underlines the importance time to treatment.
With PCI there has been recent debate as to whether there is a similar gradient of benefit over time to reperfusion as seen with fibrinolytics, or that the strategy is time insensitive. Brodie et al. (55) reviewed their extensive data on primary PCI and showed that in higher risk patients, there was indeed a gradient of benefit as a function of time (Fig. 19.8).
The use of emboli protection devices was thought to be particularly useful in MI, with the extensive clot burden and atheromatous debris that might be embolized at the time of catheter-based reperfusion. Surprisingly, the data to validate the use of such devices has not panned out. The EMERALD trial testing one such device in 501 patients without improvement in microvascular flow, reduced infarct size, or enhanced event-free survival (56). More studies are underway and the possibility of improved technology to avoid embolization at the time of the initial device deployment in the infarct vessel may occur. But no evidence currently exists to support the use of such devices at this time.
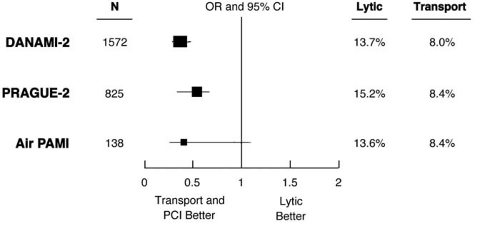 FIGURE 19.5. Odds ratio and 95% confidence intervals for the composite endpoint of death, reinfarction, and stroke at 30 days for the fibrinolytic (lytic) and transport plus coronary intervention arms in 3 randomized trials. Abbreviations: DANAMI, DANish multicenter randomized study on thrombolytic therapy versus coronary angioplasty in Acute Myocardial Infarction-2; PCI, percutaneous coronary intervention; PRAGUE-2, PRimary Angioplasty in AMI patients from General community hospitals transported to PTCA Units vs Emergency thrombolysis; Air PAMI, A randomIzed trial of transfer for Primary Angioplasty versus on-site thrombolysis in patients with high-risk Myocardial Infarction. (Source: From
Get Clinical Tree app for offline access
Topol EJ, Kereiakes DJ. Regionalization of care for acute ischemic heart disease: a call for specialized centers. Circulation
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree


|