Acquired Tricuspid and Pulmonary Valve Disease
Melvin D. Cheitlin
John S. Macgregor
Overview
Acquired disease of the tricuspid and pulmonary valves is uncommon. Tricuspid valve (TV) disease can be due to intrinsic disease of the valve or to functional tricuspid regurgitation (TR) secondary to right ventricular dysfunction. This condition, in turn, is due to intrinsic disease of the right ventricle (e.g., cardiomyopathy or coronary disease) or to pulmonary hypertension and right ventricular dilatation. Tricuspid stenosis is rare; when seen, it is often due to carcinoid heart disease rather than to rheumatic heart disease. Rarely, infective endocarditis—especially with fungal etiology—can result in tricuspid stenosis. TR owing to intrinsic valve disease is most frequently due to infective endocarditis, usually in intravenous drug users. Pulmonary stenosis is overwhelmingly congenital in etiology. Pulmonary regurgitation is rarely due to acquired organic disease of the valve, but when it occurs, it is most often iatrogenic, due to surgery on a congenitally stenotic valve. Carcinoid can involve the pulmonary valve, causing pulmonary stenosis, regurgitation, or both. Most frequently, pulmonary regurgitation is secondary to pulmonary hypertension.
TR can result in right ventricular systolic and diastolic dysfunction and right heart failure. When cardiac output falls, with a rise in systemic venous pressure, hepatic congestion, ascites, and edema can result, requiring TV surgery, either annuloplasty or replacement. Long-term results of replacement of the TV are not as favorable as with left-sided valve replacement. With tricuspid stenosis, balloon valvotomy, surgical commissurotomy, or valve replacement can be beneficial. With pulmonary stenosis, almost always congenital in etiology, balloon valvotomy has replaced surgical commissurotomy and is probably as successful in the long term. With pulmonary regurgitation, usually secondary to pulmonary hypertension, it is rarely necessary to consider pulmonary valve surgery; treatment of the pulmonary hypertension is the most beneficial approach. With medically or surgically created pulmonary regurgitation with systolic dysfunction of the right ventricle, pulmonary valve replacement is indicated.
Anatomy
The right ventricle lies anterior to the left ventricle and leftward of the right atrium. It consists of an inflow tract (including the annulus, TV, and chordal and papillary muscles) and an outflow tract (including the infundibulum of the right ventricle and the pulmonary valve). The outflow and inflow tracts are separated by four muscle bands: the infundibular septum, the parietal and septal bands of the crista ventricularis, and the moderator band. The blood supply of the right ventricle is mainly from the acute marginal branch of the right coronary artery, with small bands of right ventricle adjacent to the septum receiving blood from the posterior descending and the left anterior descending coronary arteries. In approximately 10% of hearts, the posterolateral branches of the circumflex supply portions of the posterior right ventricle. The moderator band is supplied by a branch of the first septal perforator of the left anterior
descending coronary, and the conus artery from the right coronary artery supplies the anterior wall of the right ventricle.
descending coronary, and the conus artery from the right coronary artery supplies the anterior wall of the right ventricle.
The TV arises from a fibrous annulus and has the largest circumference of all the valves: 10.0 to 12.5 cm, with an estimated valve area of approximately 7 cm2. The TV has three leaflets separated by commissures: anterior, posterior, and septal leaflets. The chordae tendineae from the anterior and posterior leaflets arise from a large anterior papillary muscle, which itself arises from the anterior wall of the right ventricle. The posterior papillary muscles are variable in number, arising from the posterior wall of the right ventricle and contributing chordae that insert on the posterior and septal leaflets. There is a conal papillary muscle arising from the septum, its chordae inserted on the anterior and septal leaflets. Frequently, these chordae arise from the septum itself, without a defined papillary muscle. The valve cusps are thin and consist of a dense collagen layer facing the right ventricle (the fibrosa) and a loose matrix composed of mucopolysaccharides (the spongiosa). The TV on the atrial surface has a variable, sparse layer of fibroelastic fibers adjacent to the annulus (1).
By Doppler echocardiography, it is possible to see a jet of TR in approximately 75% of healthy people (2), because the valve ring is so large and the leaflets are relatively narrow. Because the volume of regurgitant blood is so small and the regurgitant pressure gradients are so low, there is normally no murmur of TR. Because the tricuspid annulus forms the orifice of the right ventricular inflow tract, dilatation of the right ventricle, for any reason, worsens the failure of coaptation of the tricuspid leaflets and increases the volume of TR. With right ventricular dilatation, papillary muscles are displaced and direction of chordal pull may tether the tricuspid leaflets, worsening the TR. Any disease affecting connective tissue can be associated with TR, because the TV and chordae are composed of connective tissue.
The pulmonary valve is a semilunar valve with three cusps of equal arc similar to the aortic valve; these cusps align approximately 1.5 cm superior, anterior, and leftward of the aortic valve. The cusps have similar histologic structure to the TV, with a dense fibrosa facing the pulmonary artery and the spongiosa facing the right ventricle. Because the stress sustained by the valve is far less than that of the aortic valve, the pulmonary leaflets are thinner and more delicate. Because the pulmonary annulus acts as the root of the pulmonary artery, anything enlarging the root or increasing the diastolic pulmonary arterial pressure causes commissural separation and pulmonary valve insufficiency. Obviously, any disease altering the structure of the pulmonary leaflets also results in pulmonary valve insufficiency.
Etiology of Acquired Tricuspid Stenosis
Because the tricuspid ring is so large, there are very few diseases that can cause enough obstruction to the opening of the leaflets—either by commissural fusion, fibrosis, and calcification or by vegetative mass—to result in tricuspid stenosis. Etiologies of tricuspid stenosis are presented in Table 24.1.
In more than 90% of cases, TV stenosis is caused by rheumatic heart disease. In 92 excisions of the TV for tricuspid stenosis reported by Hauck et al (3), 85 (93%) were due to rheumatic heart disease.
Rheumatic tricuspid stenosis occurs almost always in association with mitral stenosis and is rare as an isolated finding (4). With rheumatic heart disease, the TV is involved pathologically in up to 67% of patients with mitral valve disease (5). Functionally important TR occurs in 56%, combined TR/tricuspid stenosis occurs in 41%, and isolated tricuspid stenosis occurs in only 3% (3).
TABLE 24.1 Diseases Causing Acquired Tricuspid Stenosis | |
|---|---|
|
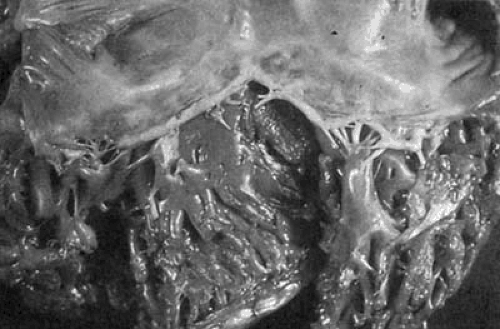
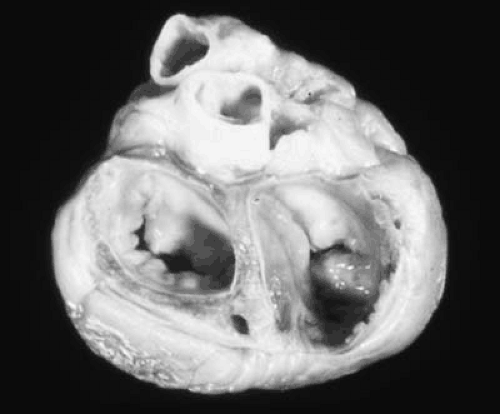
The pathology of tricuspid stenosis in rheumatic heart disease is similar to that of mitral stenosis with leaflet thickening, fibrosis and commissural fusion (3,5). An autopsy specimen demonstrating rheumatic TV disease is shown in Figure 24.1. The chordae tendineae may be thickened and fused, but less so than in mitral stenosis. Although fibrosis and retraction of the valve leaflets is common, calcification is usually absent, unlike in mitral stenosis (6). The extent of fibrosis and commissural fusion, together with chordal fusion and leaflet retraction determines whether the TV will be regurgitant, stenotic or both.
Carcinoid heart disease is the second most common etiology for tricuspid stenosis. It is almost always accompanied by TR. Carcinoid heart involvement occurs in 10% to 50% of patients with carcinoid syndrome (1,7,8,9), depending on whether the patients with isolated carcinoid tumors are included or only those with a diagnosis of metastatic carcinoid tumors. Thus, the incidence of carcinoid heart disease depends on the method of diagnosis, either by physical examination or Doppler echocardiography.
The primary site of origin for malignant carcinoid tumor is the gastrointestinal tract, including the appendix, ileum,
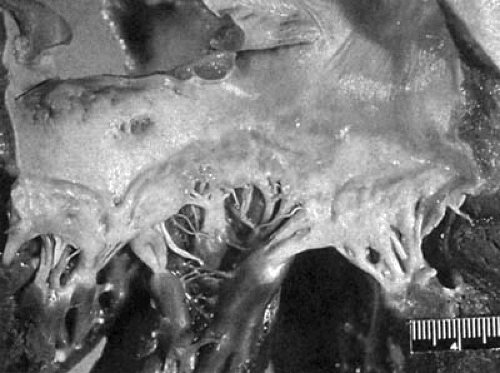
small intestine, colon, and stomach. The tumor secretes vasoactive amines, including bradykinin, 5-hydroxytryptamine (serotonin), and histamine, which are responsible for the systemic manifestations of flushing, diarrhea, and bronchospasm. Serotonin is largely detoxified in the liver by monoamineoxidase and, therefore, reaches the right heart in low concentration. However, when the carcinoid tumor metastasizes to the liver, these substances are released directly into the hepatic venous system and reach the right heart in massive concentration; there, they cause endocardial injury, resulting in the development of fibrous plaques on the endocardium and on the surfaces of the TV and pulmonary valve. The plaques are applied to, but do not destroy, the normal valve. However, the lesion can extend into the endocardium and myocardium (10). The presence of monoamineoxidase in the lung explains the lack of mitral and aortic valve involvement. The one situation where a carcinoid tumor can cause carcinoid heart disease without liver metastasis is the presence of the tumor in the testis or ovaries where venous drainage is into either the left renal vein or the inferior vena cava, thus bypassing the liver. An autopsy specimen demonstrating a TV from a patient with carcinoid heart disease is shown in Figure 24.2.
small intestine, colon, and stomach. The tumor secretes vasoactive amines, including bradykinin, 5-hydroxytryptamine (serotonin), and histamine, which are responsible for the systemic manifestations of flushing, diarrhea, and bronchospasm. Serotonin is largely detoxified in the liver by monoamineoxidase and, therefore, reaches the right heart in low concentration. However, when the carcinoid tumor metastasizes to the liver, these substances are released directly into the hepatic venous system and reach the right heart in massive concentration; there, they cause endocardial injury, resulting in the development of fibrous plaques on the endocardium and on the surfaces of the TV and pulmonary valve. The plaques are applied to, but do not destroy, the normal valve. However, the lesion can extend into the endocardium and myocardium (10). The presence of monoamineoxidase in the lung explains the lack of mitral and aortic valve involvement. The one situation where a carcinoid tumor can cause carcinoid heart disease without liver metastasis is the presence of the tumor in the testis or ovaries where venous drainage is into either the left renal vein or the inferior vena cava, thus bypassing the liver. An autopsy specimen demonstrating a TV from a patient with carcinoid heart disease is shown in Figure 24.2.
Although it has been postulated that serotonin is the agent toxic to the endocardium, only recently has more direct evidence been found to support this assumption. The functional severity of right-sided carcinoid heart disease is correlated to the increased urinary excretion of 5-hydroxyindolacetic acid, a principal metabolite of serotonin (8). Robiolio et al. (11) reported strikingly higher serotonin levels in their patients with carcinoid heart disease than in those without.
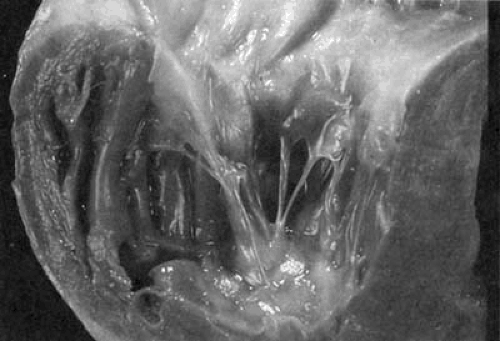
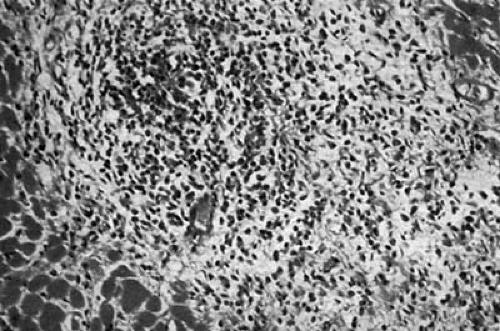
Other etiologies of TR/stenosis are much less common. Loeffler eosinophilic myocarditis can involve both ventricles causing both TR and mitral regurgitation. Occasionally, the TV is so encased that some degree of tricuspid stenosis occurs. The disease is characterized by peripheral blood eosinophilia and endocardial disease with eosinophilic infiltration, necrosis, and fibrosis, causing thrombus formation and obliteration of the ventricular cavity by scar and thrombus. The chordae and valves can be involved in this process (12,13,14). It is believed that the eosinophils produce toxic substances that cause the myocardial necrosis (15). Gross anatomic and microscopic specimens demonstrating Loeffler myocarditis are shown in Figures 24.3 and 24.4, respectively.
Finally, thrombosis or pannus formation on a prosthetic TV, calcification, and degeneration of a biologic TV can result in tricuspid stenosis (15,16,17). Occasionally, large vegetations on an infected TV can cause obstruction (18). Methysergide and pergolide can cause endocardial fibrosis similar to that seen in carcinoid syndrome. Finally, severe tricuspid stenosis with calcification of the TV can be related to chronic trauma and has been reported in patients with permanently indwelling catheters across the TV for ventriculoatrial shunts (19), peritoneal venous shunts (20), and pacemaker leads (21).
Pathophysiology
When the TV becomes stenotic by fibrosis, disease, or commissural fusion, the blood flow from systemic veins and the right
atrium into the right ventricle is obstructed; with atrial contraction against this stenotic valve, a large ‘a’ wave is seen. A pressure gradient develops between the right atrium and right ventricle in diastole. This results in a low-pitched diastolic rumbling murmur that is generated by the entire stroke volume crossing the obstructive valve under a low-pressure gradient. The gradient can be accentuated in inspiration, when there is an increase in systemic venous return to the right atrium and a drop in right ventricular filling pressure, and decreased in expiration; therefore, the murmur may become louder during inspiration and decrease in expiration. If the valve is flexible, a high-pitched tricuspid opening snap may be generated at the time of the opening of the TV (22). With severe tricuspid stenosis, there is a drop in cardiac output. With the elevation of pressure in the right atrium, right atrial dilatation occurs. With increasing right atrial pressure, systemic venous pressure, and systemic capillary pressure, edema develops, first in the dependent areas (e.g., the ankles) and then progressively advancing to the thighs, abdomen, and thorax. Finally, edema advances to the face, a condition known as anasarca. There is also the development of ascites and pericardial effusions (4,23).
atrium into the right ventricle is obstructed; with atrial contraction against this stenotic valve, a large ‘a’ wave is seen. A pressure gradient develops between the right atrium and right ventricle in diastole. This results in a low-pitched diastolic rumbling murmur that is generated by the entire stroke volume crossing the obstructive valve under a low-pressure gradient. The gradient can be accentuated in inspiration, when there is an increase in systemic venous return to the right atrium and a drop in right ventricular filling pressure, and decreased in expiration; therefore, the murmur may become louder during inspiration and decrease in expiration. If the valve is flexible, a high-pitched tricuspid opening snap may be generated at the time of the opening of the TV (22). With severe tricuspid stenosis, there is a drop in cardiac output. With the elevation of pressure in the right atrium, right atrial dilatation occurs. With increasing right atrial pressure, systemic venous pressure, and systemic capillary pressure, edema develops, first in the dependent areas (e.g., the ankles) and then progressively advancing to the thighs, abdomen, and thorax. Finally, edema advances to the face, a condition known as anasarca. There is also the development of ascites and pericardial effusions (4,23).
History and Physical Examination
In patients with tricuspid stenosis, the history depends on the severity of the tricuspid stenosis and the symptoms associated with the etiology and concomitant cardiac lesions. Therefore, patients with rheumatic heart disease almost always have concomitant mitral valve disease, usually mitral stenosis. The symptoms of shortness of breath, orthopnea, and paroxysmal nocturnal dyspnea due to the mitral stenosis may dominate the patient’s symptomatology (23). Decrease in cardiac output in the patient with severe mitral stenosis results in a lower-than-expected left atrial pressure and protects against pulmonary congestion (24).
In metastatic carcinoid tumors, the symptoms of flushing and diarrhea may be the most impressive symptoms. In the patient with mitral stenosis, atrial fibrillation and its concomitant palpitations may occur.
From the tricuspid stenosis alone, the symptoms alone are increased fatigability owing to a decreased cardiac output and the effects of fluid retention and elevated systemic venous pressure. Edema—in the most severe cases, anasarca—may be the patient’s major complaint. Ascites and abdominal enlargement, hepatic distention, and right upper quadrant discomfort may dominate the history.
Physical findings owing to tricuspid stenosis are an abnormally large ‘a’ wave in the jugular venous pulse. The ‘a’ wave occurs at the time of the first heart sound and is rapid rising, resembling a carotid arterial pulse. The difference is that the ‘a’ wave can be eliminated by firm pressure on the jugular vein, whereas an arterial pulse cannot. The ‘v’ wave, without accompanying TR, is inconspicuous, and the ‘y’ descent is slow or absent (24).
The classic finding is that of a low-pitched diastolic murmur, usually along the left sternal border in the third and fourth intercostal spaces (25). In sinus rhythm, it is most prominent at end diastole, whereas in atrial fibrillation, it is usually more prominent in early and mid-diastole. If the valve is flexible, an opening snap can be heard along the lower left sternal border, although this is more unusual than the opening snap heard with mitral stenosis. If the right atrium is very large, an impulse can be felt in diastole along the lower right sternal border. With pure tricuspid stenosis, there is no right ventricular lift.
Because mitral stenosis is almost always present when tricuspid stenosis is due to rheumatic heart disease, the physical findings of mitral stenosis (the diastolic murmur, the loud first heart sound, and opening snap at the apex) are more conspicuous than the findings of tricuspid stenosis. However, tricuspid stenosis should be suspected when mitral stenosis is present when the elevation in the jugular venous pressure is greater than the signs of pulmonary congestion and the patient complaints of dyspnea, orthopnea, and paroxysmal nocturnal dyspnea. The accentuation with inspiration of a diastolic murmur along the lower left sternal border is known as the Rivero-Carvallo sign; it is characteristic of tricuspid stenosis (26), even in the presence of mitral stenosis, in which inspiration does not affect the loudness of the murmur. The murmur can also be increased at the bedside by increasing venous return by passively lifting the legs (27). Also, the presence of a large ‘a’ wave in the jugular venous pulse in the presence of mitral stenosis should raise suspicion of concomitant tricuspid stenosis. Because of the elevation in systemic venous pressure, there may be hepatomegaly, edema, or ascites. If the tricuspid stenosis is due to a metastatic carcinoid tumor, then a large nodular liver is frequently present, and a ruddy cyanotic flush of the face may be seen periodically.
Laboratory Findings
Chest X-Ray
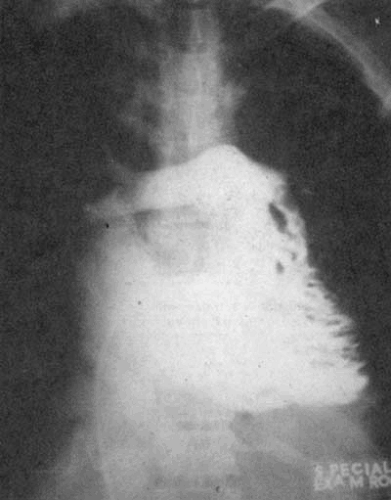
Cardiomegaly owing to right atrial enlargement is frequent. The superior vena cava and azygous vein may be prominent. With pure tricuspid stenosis, the left atrium and right ventricle are not enlarged, and the pulmonary vascular markings are normal or decreased (24). With concomitant mitral stenosis, there is left atrial enlargement, although signs of pulmonary congestion are less prominent and without tricuspid stenosis.
Electrocardiogram
A large, peaked P wave in lead II is consistent with right atrial abnormality. A peaked, broad, notched, or biphasic P wave with terminal negative potential in the V1 lead is consistent with left atrial abnormality and may be present, especially if there is concomitant mitral stenosis. Atrial arrhythmias, especially
atrial fibrillation, are often present with severe tricuspid or mitral stenosis.
atrial fibrillation, are often present with severe tricuspid or mitral stenosis.
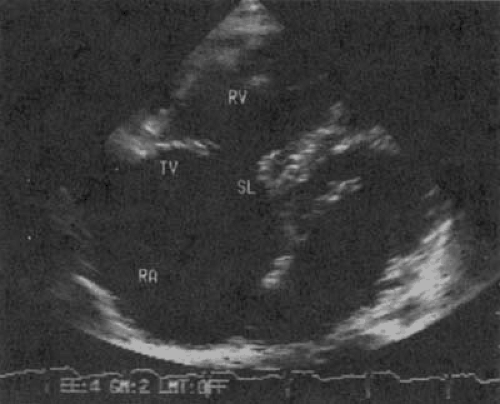
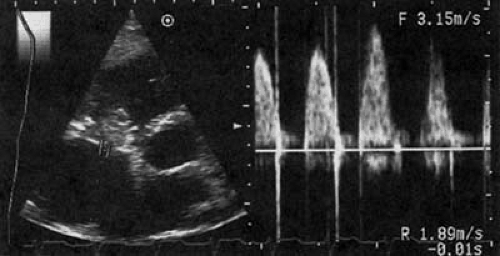
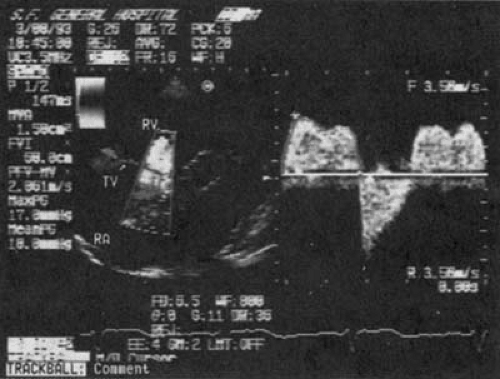 FIGURE 24.6. Doppler examination of the patient in Figure 24.5 with carcinoid heart disease and tricuspid stenosis and regurgitation. The peak gradient is calculated to be 18 mm Hg, and the mean gradient is 10 mm Hg. Abbreviations: RA, right atrium; RV, right ventricle; TV, tricuspid valve. (Source: From Cheitlin MD, MacGregor JS. Acquired tricuspid and pulmonic valve disease. In: Rahimtoola SH, Braunwald E, eds. Atlas of heart diseases. Vol. 11: Valvular heart disease. St. Louis: Mosby, 1997 , with permission.) |
Doppler and Two-Dimensional Echocardiography
The findings on Doppler echocardiography depend on the etiology of the tricuspid stenosis. With rheumatic heart disease, the TV is domed in diastole. The leaflets are often thickened, with decreased mobility owing to fusion along the commissures by the inflammatory process. The chordae tendineae may appear shortened and thickened. There is increased flow velocity across the TV in diastole; from this, by the modified Bernoulli equation, the diastolic gradient can usually be accurately estimated. With M-mode echocardiography, the E–F slope of the anterior leaflet is reduced, and there is increased reflectance from the valve. If rheumatic mitral stenosis or mitral regurgitation is present, the characteristic echocardiographic findings of these diseases can also be seen.
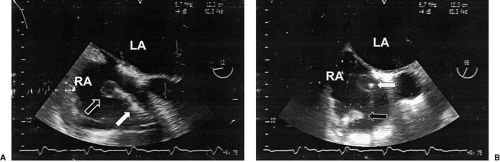
With carcinoid involvement, the valve is thickened and rigid, frequently fixed in the open position and acting like a washer, neither opening nor closing. Most often, TR is the predominant finding, along with an increase in diastolic velocity across the valve, with a reduced E–F slope that is consistent with tricuspid stenosis (7,28) (Figs. 24.5 and 24.6.). Echocardiography of stenotic bioprosthetic valves in the tricuspid position shows increased diastolic velocity, abnormal reflectance from the biologic leaflets, and occasionally, calcification. Thrombus and vegetations from infective endocarditis can cause functional
stenosis of a bioprosthetic valve. A large, obstructive vegetation on a bioprosthetic valve is shown in Figure 24.7.
stenosis of a bioprosthetic valve. A large, obstructive vegetation on a bioprosthetic valve is shown in Figure 24.7.
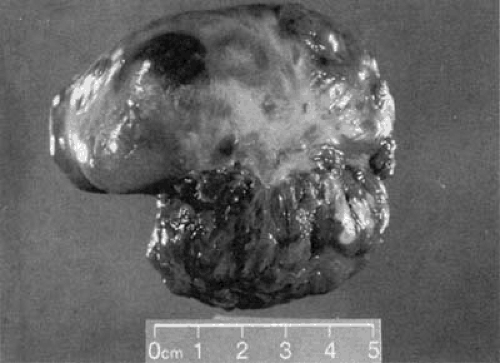 FIGURE 24.9. Gross anatomic specimen removed at surgery from the patient described in Figure 24.8. The tumor was adherent to the superior wall of the right atrium and measured approximately 8 cm in its longest dimension. |
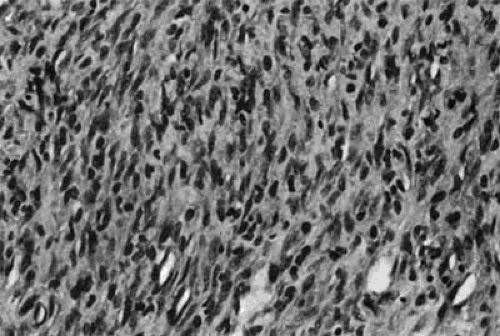 FIGURE 24.10. Microscopic section of the tumor described in Figure 24.8, stained with hematoxylin–eosin. The tumor was identified as a rhabdomyosarcoma. |
Echocardiography is especially helpful in identifying those diseases that can simulate tricuspid stenosis, such as right atrial tumor, either growing up from the inferior vena cava across the TV (e.g., renal carcinoma) or from a right atrial myxoma obstructing diastolic flow across the TV. A two-dimensional echocardiogram demonstrating a large tumor that almost totally obstructs the TV is shown in Figures 24.8, 24.9, and 24.10 show the gross anatomic and microscopic features of this tumor, which was found to be a rhabdomyosarcoma.
Cardiac Catheterization
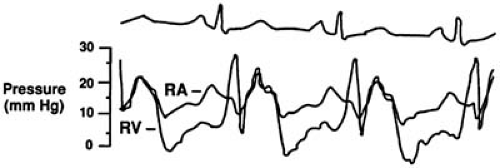
At present, the diagnosis and estimation of severity of tricuspid stenosis are made by physical examination and confirmed by Doppler echocardiography. Cardiac catheterization is usually not necessary. When catheterization is done, the right atrial pressure is characterized by a large ‘a’ wave with an inconspicuous ‘v’ wave and a slow ‘y’ descent. The cardiac output is low, and the right ventricular and pulmonary artery pressures are normal or low. The TV area is decreased, and clinical tricuspid stenosis does not occur until the valve area is reduced to approximately 1.5 cm2. Depending on the cardiac output, the diastolic gradient can be quite low (3–5 mm Hg) and should be measured with simultaneous catheters in the right atrium and right ventricle (22). An example of right atrial and right ventricular pressure tracings in a patient with carcinoid heart disease and tricuspid stenosis is shown in Figure 24.11. The gradient is accentuated by increasing the cardiac output and decreasing the diastolic filling period by exercising, lifting the legs, and increasing the heart rate with atropine. Because the gradient is so low, gradients estimated by pullback of the catheter across the TV from the right ventricle to the right atrium can miss significant tricuspid stenosis.
Differential Diagnosis of Tricuspid Stenosis
Any disease that delays emptying of the right atrium in diastole can simulate tricuspid stenosis; this includes right atrial myxoma, which can prolapse into the tricuspid orifice in diastole and obstruct diastolic flow across the valve. In this situation, an early diastolic sound, or “tumor plop,” may simulate the opening snap of tricuspid stenosis. A tumor growing up from the inferior vena cava (e.g., renal cell carcinoma) can involve the TV and simulate tricuspid stenosis. Other intracardiac tumors, including lymphoma and sarcoma, can obstruct TV flow and mimic tricuspid stenosis. Constrictive pericarditis can involve primarily the right ventricle and simulate tricuspid stenosis (29).
Etiology of Tricuspid Regurgitation
In contrast to tricuspid stenosis, TR can be due to a number of different etiologies. Diseases affecting any part of the tricuspid apparatus can result in TR, including pathology of the annulus, leaflets, chordae, and papillary muscles of the right ventricular wall.
The etiologies of TR can be classified as (a) diseases causing pulmonary hypertension and right ventricular failure with tricuspid annular dilatation, or (b) primary diseases of the tricuspid apparatus (30). Table 24.2 lists diseases under each group. The most common etiology for TR is failure of the right ventricle due to pulmonary hypertension caused by left heart failure. Common causes of left heart failure are hypertension, coronary artery disease, mitral and aortic valve disease, and cardiomyopathy. Diseases causing an increase in pulmonary vascular resistance and pulmonary hypertension are listed in Table 24.2. The latest additions to this list have been acquired immunodeficiency syndrome (31) and anorexic drugs (e.g., fenfluramine with or without phentermine and dexfenfluramine) (32).
TABLE 24.2 Diseases Causing TR | ||
|---|---|---|
|
The pathologic changes of the TV seen in rheumatic heart disease, carcinoid heart disease, and eosinophilic myocarditis have been described. Endomyocardial fibrosis is a rare disease found mainly in women. It is characterized by endocardial fibrosis that affects mainly the left ventricle (but also the right ventricle) and may involve the atrioventricular valves, causing mitral regurgitation or TR. It is also characterized by mural thrombus, which can obliterate the ventricular apices (33). With TR, there is thickening, shortening, and increased stiffness of the valve without significant commissural fusion (34). With pulmonary hypertension, the tricuspid leaflets and chordae tendineae are normal. There is right ventricular hypertrophy, dilatation of the right ventricle and tricuspid annulus, and TR. Malalignment and decreased force of contraction of the papillary muscles are also factors (35). With right ventricular infarction—which is almost always associated with diaphragmatic wall infarction because the acute marginal artery from the right coronary artery is the major source of blood supply to the anterior wall of the right ventricle—there is infarction and, later, fibrosis of the right ventricular papillary muscles and dilatation of the right ventricle (36,37). With right ventricular cardiomyopathy or dysplasia and Uhl disease, which may be the most severe form of right ventricular dysplasia, the patient can present with right ventricular failure and TR (38). Myxomatous changes in the TV are similar to changes seen in the mitral valve, with thickening of the spongiosa and undermining support of the chordae tendineae insertions (3). The
floppy TV is seen almost exclusively in the presence of a myxomatous prolapsing mitral valve. The incidence of floppy TV in an autopsy population is as high as 3.2% of consecutive autopsies (39). An example of a myxomatous TV is shown in Figure 24.12. With connective tissue diseases like Marfan syndrome, there can be dilation of the tricuspid annulus, prolapsing valve leaflets, and elongated chordae (40).
floppy TV is seen almost exclusively in the presence of a myxomatous prolapsing mitral valve. The incidence of floppy TV in an autopsy population is as high as 3.2% of consecutive autopsies (39). An example of a myxomatous TV is shown in Figure 24.12. With connective tissue diseases like Marfan syndrome, there can be dilation of the tricuspid annulus, prolapsing valve leaflets, and elongated chordae (40).
Penetrating trauma can puncture the leaflets, rupture the chordae, or sever the papillary muscles (41). With blunt trauma (most frequently owing to motor vehicle accidents), the right ventricle is the most common site of myocardial contusion. If right ventricular myocardial contusion is extensive, right ventricular failure and dilatation occur. With nonpenetrating injury (usually compressive injury or deceleration injury), complete fracture of the papillary muscles is not uncommon (42). A right ventriculogram from a patient with traumatic rupture of a tricuspid papillary muscle with resultant TR is shown in Figure 24.13. Permanent pacemaker and implantable cardioverter-defibrillator leads have been reported to cause right heart failure owing to severe TR (43).
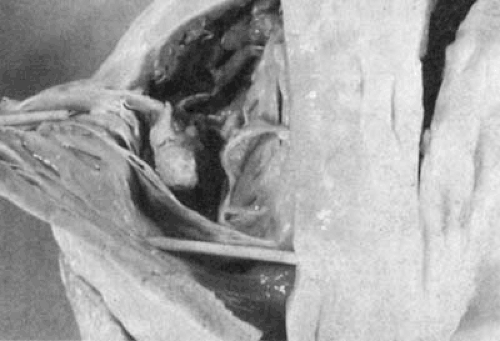
Infective endocarditis is seen almost exclusively in intravenous drug users and, occasionally, in patients who have trauma to the TV from the jet of a ventricular septal defect or an indwelling catheter across the TV (Fig. 24.14). Chronic alcoholism and hereditary immunodeficiency disease are also associated with an increased incidence of tricuspid endocarditis (44). Staphylococcus aureus is the most common organism seen. Hecht and Berger (44) reported S aureus to be responsible for tricuspid endocarditis in 82% of 132 cases seen in intravenous drug users. An autopsy specimen demonstrating a large vegetation on the TV is shown in Figure 24.15.
Congenital heart lesions (e.g., Ebstein anomaly, primary TR, and cleft leaflets with endocardial cushion defects) are developmental defects due to malformation of the TV with displacement of the attachment of septal and posterior leaflets into the body of the right ventricle (e.g., Ebstein anomaly) (30), tethering of the tricuspid leaflets (e.g., congenital non-Ebstein primary TR), or abnormal formation of the leaflets and clefting (e.g., endocardial cushion defects). Congenital lesions of the TV are not discussed further in this chapter.
Pathophysiology of Tricuspid Regurgitation
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree