Atrial septal defects (ASDs) account for approximately 7% to 10% of all congenital heart disease. Secundum defects, which comprise 60% to 75% of all ASDs, are the most common. Two-dimensional, Doppler, and color Doppler echocardiography play a key role in confirmation of the clinical diagnosis of an ASD, providing definitive evaluation of abnormalities of atrial septation. An ASD may exist in isolation or in association with other congenital cardiac abnormalities. It is important to recognize abnormalities of atrial septation, as these defects may affect clinical management, such as whether catheter-based or surgical treatment is preferred. Cor triatriatum, a very rare type of septation abnormality, will also be reviewed. Primum ASD, which is within the spectrum of atrioventricular septal defects, will be considered in a separate chapter.
ATRIAL SEPTAL ANATOMY AND EMBRYOLOGY
A defect in the atrial septum results in a direct communication between the left atrium (LA) and right atrium (RA). To characterize the location of ASDs, an understanding of septal anatomy is critical. An ASD is typically classified by its location in relationship to the fossa ovalis (Fig. 6.1). During embryologic development, the primitive atrium undergoes a complex septation process. The septum primum extends inferiorly from the middle of the atrium toward the region of the endocardial cushions, initially leaving an opening called the ostium primum. The inferior portion of the septum primum subsequently fuses with the developing endocardial cushions to close the inferiorly located ostium primum (see Fig. 6.1A–B). Tissue reabsorption (or programmed cell death) in the middle of septum primum leads to a second, central opening, or ostium secundum. Concurrently, development of the septum secundum occurs, and once joined with the endocardial cushions, the inferior portions of the two atria are separated. The remaining defect in septum secundum is the foramen ovale, which allows flow from the fetal RA to LA during gestation. Once birth occurs, fusion of these two septa should occur, functionally closing the foramen ovale; however, probe patency is present in approximately 25% to 30% of the population. Secundum ASDs, typically occurring in the central or secundum portion of the atrial septum, actually result from a true deficiency in the septum primum (see Fig. 6.1D).
Both venae cavae (superior and inferior) and the coronary sinus (CS) enter the more posterior smooth-walled portion of the RA. Defects within this superior/posterior portion of the atrial septum are termed sinus venosus defects (see Fig. 6.1D) and result from an abnormality of right pulmonary vein incorporation into the developing atrium. These defects typically occur in conjunction with an abnormality of pulmonary venous return from the right lung. Geva and Van Praagh propose that sinus venosus ASD is not a true ASD but an unroofing of the right pulmonary vein into the superior vena cava (SVC) with the interatrial communication being the left atrial orifice of the unroofed pulmonary veins.
A CS defect, or unroofed CS syndrome, results from an abnormality in the development of the left atrioventricular fold. In this spectrum of abnormalities, complete or partial unroofing of the CS, with direct communication to the LA, is present and is uniformly associated with a left SVC (see Fig. 6.1D).
Cor triatriatum sinister, another very rare form of abnormal atrial septation, occurs within the LA. Cor triatriatum may result from abnormal incorporation of the common pulmonary vein into the LA, causing obstruction between the pulmonary vein confluence and the LA; however, not all variants of this rare lesion are embryologically consistent with this theory.
ATRIAL SEPTAL DEFECT
Clinical Presentation
Clinical presentation of the patient with an ASD varies with patient age, size of the defect, and the presence of associated lesions. In the infant with an isolated ASD, presentation with symptomatic pulmonary overcirculation is unusual, as right ventricular (RV) compliance is low and atrial shunting may be minimal early in life. An ASD may be found as part of complete evaluation of the infant with other cardiac anomalies. As right ventricular (RV) compliance improves during infancy and early childhood, left-to-right shunting at the atrial level typically increases. The child or adolescent with a hemodynamically significant ASD usually presents with a cardiac murmur or, less commonly, with symptoms of exercise intolerance or fatigue. The physical examination characteristically reveals a hyperdynamic RV impulse in a patient with a large shunt. A systolic ejection murmur is heard over the left sternal border in association with a widely fixed split second heart sound. A diastolic flow rumble over the tricuspid valve inflow area is present with a significant shunt at the atrial level due to the large volume of blood returning through the tricuspid valve. The chest radiograph may show cardiomegaly with increased pulmonary vascular markings in addition to prominence of the main pulmonary artery segment and central pulmonary arteries.
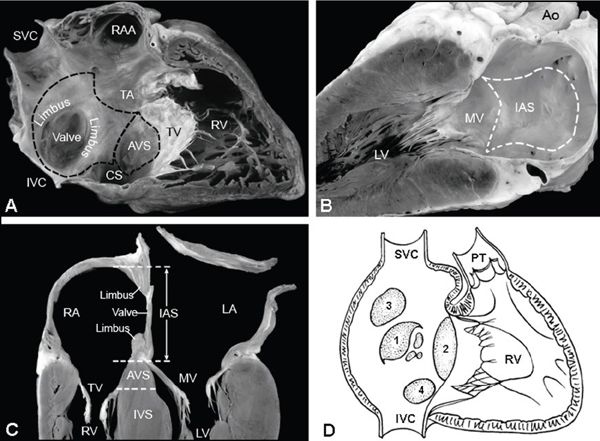
Figure 6.1. Atrial septal anatomy. A: Right-sided, two-chamber view of right atrium (RA) and right ventricle (RV). The interatrial septum (IAS) is outlined (dotted line); the superior limbus and inferior limbus surround the valve of the fossa ovalis. The atrioventricular septum (AVS) is shown just above the tricuspid valve. B: Left-sided, two-chamber view of left atrium with IAS depicted within the dotted line. C: Four-chamber view of the crux of the heart, demonstrating IAS (between the RA and LA) with superior limbus, inferior limbus, and valve of the fossa ovalis. The AVS lies between the RA and the left ventricle (LV). D: Diagrammatic representation of the different types of atrial septal defects (ASDs), numbered in decreasing frequency according to rates of occurrence: secundum ASD (1), primum ASD (2), sinus venosus ASD (3), coronary sinus ASD (4). CS, coronary sinus; IVC, inferior vena cava; IVS, interventricular septum; MV, mitral valve; PT, pulmonary trunk; RAA, right atrial appendage; SVC, superior vena cava; TV, tricuspid valve. (Reprinted by permission of Mayo Foundation.)
Atrial level shunting tends to increase with age. The older patient may present with more significant symptoms of exercise intolerance or fatigue and very rarely with overt right heart failure. Atrial arrhythmias are uncommon before adulthood but may persist following late repair unless a surgical arrhythmia procedure is performed concurrently. Pulmonary hypertension or pulmonary vascular obstructive disease is uncommon in the setting of an isolated ASD but must be evaluated carefully before treatment recommendations can be made.
Physiology
Communication between the LA and the RA allows oxygenated pulmonary venous blood to enter the RA via the defect in the atrial septum. Left atrial pressure is typically higher than right atrial pressure through most of the cardiac cycle, so the predominant shunt is left to right. A very transient right-to-left shunt is commonly observed due to variability in atrial compliance. A patent foramen ovale (PFO) may allow right-to-left shunting and the potential for paradoxical embolism to the systemic circulation. Most commonly in the setting of an ASD, left-to-right shunting at the atrial level produces right-sided volume overload, causing progressive RA and RV enlargement and pulmonary overcirculation. In the patient with pulmonary vascular disease or RV outflow obstruction, right-to-left shunting at the atrial level may be significant as RV compliance worsens over time.
A patient with a CS ASD and connection of the left SVC to the roof of the LA may have variable degrees of cyanosis due to mixing of systemic venous return with the oxygenated pulmonary venous return. The amount of desaturation is proportional to the amount of systemic venous blood carried by the left SVC.
Complications
A small ASD will typically undergo spontaneous closure (complete or nearly complete) and should not require treatment or produce long-term complications. A moderate (6 to 12 mm) or large (greater than 12 mm) ASD may not cause significant symptoms or complications early in life, but as left ventricular compliance decreases over time the volume of intracardiac shunt will steadily increase. It is unlikely that a defect larger than 8 mm will undergo spontaneous closure; in fact, the anatomic size of a moderate or large ASD may increase over time. Also, chronic RV volume overload may produce tricuspid annular dilation, resulting in progressive tricuspid valve regurgitation. Longstanding right atrial dilation contributes to an increased risk of atrial arrhythmias. Although atrial flutter and atrial fibrillation are uncommon in childhood, the prevalence increases with age in a patient with untreated or undiagnosed ASD. Arrhythmias may persist after repair, particularly with repair at an older age or in the setting of elevated pulmonary artery pressure or resistance. Natural history studies suggest that repair of a hemodynamically significant ASD before age 25 years should allow a normal life expectancy.
Pulmonary hypertension and pulmonary vascular obstructive disease in association with ASD are uncommon, occurring in a small percentage of patients. Additional complications of right-to-left shunting may include paradoxical emboli and risk of stroke. Less commonly seen in the setting of unroofed CS is cerebral embolization and risk of brain abscess.
EVALUATION OF ATRIAL SEPTAL DEFECT: OVERVIEW
Right-sided chamber enlargement is the hallmark of an atrial level shunt. When seen on echocardiographic imaging, this should prompt a comprehensive evaluation of the atrial septum and definition of pulmonary venous return.
Because the atrial septum is a complex three-dimensional structure, it must be imaged from multiple planes. Portions of the atrial septum may be quite thin and difficult to visualize; therefore, definitive imaging of the atrial septum with transthoracic echocardiography can be challenging, particularly in the older adolescent or adult. Orthogonal views are needed in order to resolve tissue rims. Diagnostic images are typically obtained with the imaging transducer perpendicular to the structures of interest, so as to not produce artificial dropout or the appearance of a defect where there is not an ASD. The echocardiographer needs to provide an assessment of defect location, size, and distance from surrounding cardiac structures. In addition, a complete assessment of potential associated cardiac abnormalities is indicated. Complete spectral Doppler and color Doppler assessment is important in evaluation of the primary defect as well as to provide hemodynamic evaluation, including estimation of RV systolic and pulmonary artery pressure and assessment of tricuspid regurgitation. Noninvasive shunt quantification is not typically performed.
The goals of imaging should be to provide definitive information for the clinician, to assess the hemodynamic significance of the defect(s), and to aid in planning for catheter-based intervention or surgical treatment. If image quality from the transthoracic approach is suboptimal and the echocardiographer suspects an atrial level shunt or abnormality of pulmonary venous return, then transesophageal echocardiography is warranted. In some cases, cardiac magnetic resonance imaging (MRI) may be needed to definitively evaluate abnormalities of pulmonary venous return, particularly if the abnormal connection is high in the superior vena cava.
Subcostal Imaging
Subcostal imaging is ideal for evaluation of the atrial septum when image quality is adequate. From this orientation, the transducer beam is relatively perpendicular to the atrial septum in both the four-chamber (coronal) and short-axis (sagittal) views, so that tissue structures can be resolved to be sure that artifactual dropout is not present. Key echo findings include the presence of true dropout in the atrial septum, and right-sided structural enlargement (RA, right ventricle, and pulmonary artery). The edges of the defect may produce additional reflections known as a “T-artifact,” allowing identification of defect rims.
Subcostal Four-Chamber (Coronal) Views
Four-chamber or coronal plane imaging provides images of the atrial septum along an anterior-to-posterior axis and is useful to identify the location and size of an ASD. Imaging is carried out with transducer sweeps from anterior to posterior, providing evaluation of the ASD in the long axis of the defect (Fig. 6.2A) (Video 6.1). The location of the defect in the septum and its relationship to the right-sided SVC and right upper pulmonary vein should be ascertained (Fig. 6.2B). Scanning anteriorly and superiorly provides evaluation of the atrial septum immediately behind the aorta. The posterior-inferior portion of the atrial septum can be evaluated by angling the transducer more posteriorly in the four-chamber plane, but artifactual dropout may occur. Short-axis imaging is preferred for evaluation of posterior and inferior rims. In the normal heart, the atrial septum should be seen inferiorly/posteriorly extending to the level of the atrioventricular valves.
The addition of color Doppler typically reveals a continuous left-to-right shunt (Fig. 6.2C), although a transient, brief amount of right-to-left shunting may occur with variations in respiratory cycle. Pulsed-wave Doppler at the level of the ASD will demonstrate low-velocity, phasic, left-to-right flow; very transient right-to-left flow may be detected. Lack of phasic flow or increased velocity with continuous flow suggests a significant gradient between the LA and RA caused by a restrictive defect (depending on the direction of flow).
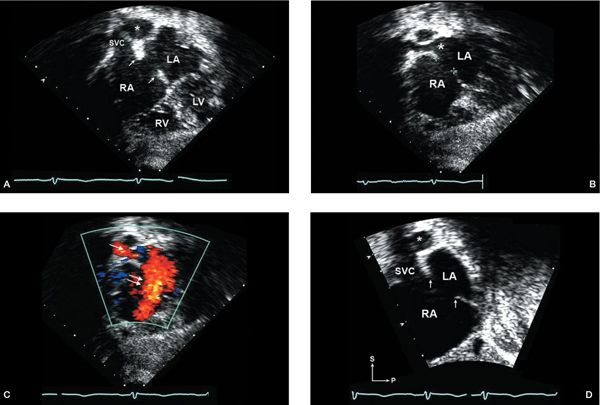
Figure 6.2. Subcostal imaging of a moderate-sized secundum atrial septal defect (ASD). A: Subcostal coronal plane focused on biatrial view, showing dropout in mid-septum between the left atrium (LA) and right atrium (RA) consistent with moderate-sized secundum ASD. The rims of the defect in this plane appear well developed (single arrows, superior edge; double arrow, inferiorly). The right pulmonary artery (asterisk) can be seen just posterior to the superior vena cava (SVC). B: Slight anterior angulation of the transducer from the coronal view (shown in A) will bring into view the right upper pulmonary vein (large asterisk) entering the LA. C: Addition of color Doppler shows left-to-right shunt through the ASD (double arrow) and the right pulmonary vein flow to the LA (single arrow). D: Rotating the transducer approximately 90 degrees from coronal view provides a short-axis (sagittal) view, demonstrating the secundum ASD with adequate superior (near the SVC) and inferior/posterior rims (single arrows) for consideration of device closure. (Imaging note: Farther rightward movement of the transducer to achieve a bicaval view will show the entrance of the inferior vena cava into the RA anterior to the inferior rim.) LV, left ventricle; RV, right ventricle.
Subcostal Short-Axis (Sagittal) Views
Subcostal short-axis (SAX) imaging is obtained in an orthogonal imaging plane to the four-chamber (coronal) view, providing imaging in a superior-inferior axis (Fig. 6.2D and Video 6.2). Sweeping the transducer from right to left allows evaluation of the relationship of the right-sided SVC, pulmonary veins (particularly right), and the superior/inferior rims of an ASD. One must be careful to note the position of the Eustachian valve, which is located anteriorly at the entrance of the inferior vena cava to the RA; this should not be confused with a rim of the ASD. Short-axis imaging allows imaging of the anterior-superior and posterior-inferior atrial septum. In addition, excellent images of the right ventricle (RV), RV outflow tract (RVOT), and pulmonary valve can be obtained from the subcostal short-axis plane. Doppler interrogation can be performed to evaluate RVOT velocities to determine if a significant outflow tract gradient exists.
Parasternal Views
Parasternal Long-Axis View
Characteristic signs of RV enlargement, volume overload, and paradoxical septal motion are seen in the parasternal long-axis plane when there is a significant atrial level shunt (Fig. 6.3A). Mitral valve prolapse, seen in association with ASD, is best demonstrated in the long-axis plane. Angling the transducer inferiorly toward the tricuspid inflow view may demonstrate color flow across the atrial septum, although this view is often insufficient for visualization of an ASD. Color Doppler should also be used to evaluate tricuspid valve regurgitation, and, if present, pulsed-wave Doppler can be used to obtain an estimation of RV systolic pressure (RVSP).
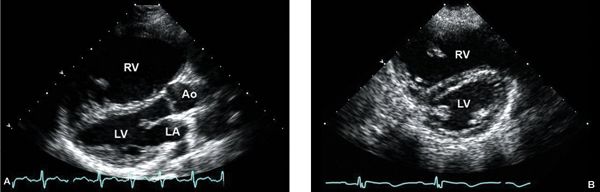
Figure 6.3. A: Parasternal long-axis view with severe right ventricular (RV) enlargement due to a large secundum. B: Parasternal short-axis view demonstrating RV enlargement and diastolic flattening of the interventricular septum, consistent with RV volume overload. Ao, aorta; LA, left atrium; LV left ventricle.
Angling the transducer superiorly toward the left shoulder will demonstrate the RVOT, the pulmonary valve leaflets, and main pulmonary artery. Dilation of the main pulmonary artery is seen with significant atrial level shunting. The pulmonary valve leaflets should be examined for morphology and function. In the RVOT, pulsed-wave Doppler velocities as high as to 2.5 m/s may be seen with large atrial level shunts; velocities greater than 2.5 m/s suggest an additional component of valvular pulmonary stenosis. Doming or restricted motion of the pulmonary valve will be seen with valve stenosis and should be evaluated carefully with two-dimensional imaging for correlation with Doppler findings.
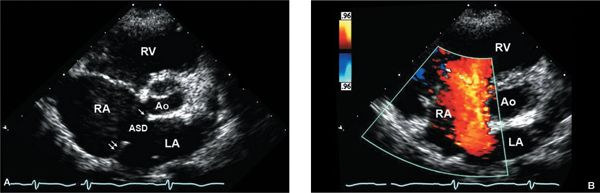
Figure 6.4. Parasternal short-axis view of large secundum atrial septal defect (ASD). A: Right atrial (RA) and right ventricular (RV) enlargement are consistent with atrial level shunting. RA is significantly more enlarged than the left atrium (LA). The diminutive anterior, retroaortic rim (single arrow) of the ASD is well visualized in this image. The posterior rim is well demonstrated in this view (double arrow), as it is not parallel to the imaging plane. Ao, aorta. B: The addition of color Doppler shows a large shunt from LA to RA consistent with the secundum ASD.
Parasternal Short-Axis View
Parasternal short-axis imaging is also very useful for evaluating RV volume overload and flattening or paradoxical motion of the interventricular septum (Fig. 6.3B). Diastolic flattening of the septum is common with volume overload; systolic flattening is seen with pressure overload. Doppler evaluation of tricuspid regurgitation, including assessment of RVSP, is important for comprehensive hemodynamic assessment and to rule out possible coexistent pulmonary hypertension. RA enlargement is also appreciated from this view. As the atrial septum is somewhat perpendicular to this plane of imaging, septal rims may be evaluated accurately (Fig. 6.4A and Video 6.3). Color Doppler will show left-to-right shunting from LA to RA in a typical secundum ASD (Fig. 6.4B). The RVOT, pulmonary valve, and main pulmonary artery are well seen from the SAX view. Anatomic evaluation of the infundibulum and pulmonary valve is also recommended to correlate with Doppler findings.
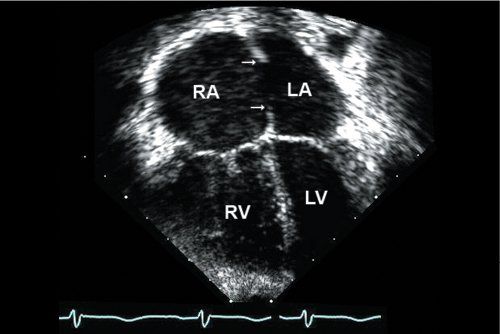
Figure 6.5. Apical four-chamber view shows moderate right atrial (RA) and right ventricular (RV) enlargement. There is a large secundum atrial septal defect (ASD) with well-developed superior rim and inferior rims (single arrows). (Imaging note: One must be careful to define the rims of tissue carefully, as they are typically more parallel to the plane of imaging from the apex and artificial dropout should be avoided.)
Apical Four-Chamber View
RA and RV enlargement can be easily seen from the apical four-chamber orientation (Fig. 6.5). One should use color Doppler to evaluate the degree of tricuspid regurgitation and pulsed-wave Doppler to provide an accurate estimation of RVSP (Fig. 6.6) [Video 6.4]. Associated mitral valve abnormalities can also be evaluated, taking care to rule out mitral valve stenosis that may accentuate an atrial level shunt due to elevation of left atrial pressure. Because the four-chamber imaging plane is parallel to the atrial septum, the suggestion of dropout in the thinner mid-portion of the atrial septum must be confirmed from additional views. The apical four-chamber view can be used for evaluation of the inferior-most portion of the atrial septum at the level of the crux of the heart.
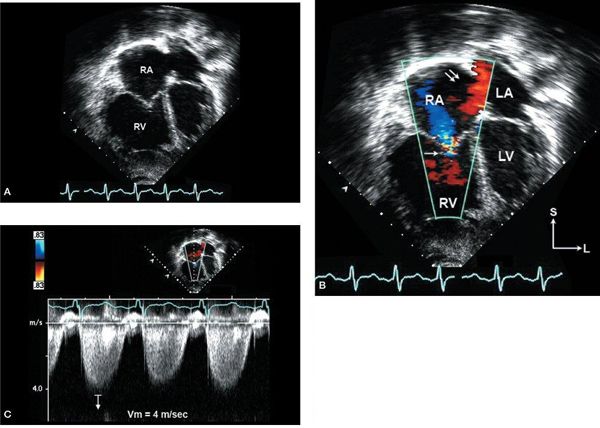
Figure 6.6. Apical four-chamber images in a patient with pulmonary hypertension and moderate secundum atrial septal defect (ASD). A: Severe right atrial (RA) and right ventricular (RV) enlargement with right ventricular hypertrophy is seen. B: Left-to-right shunt is seen through the ASD (double arrows) with associated tricuspid valve regurgitation (single arrow). C: Quantitative hemodynamic assessment with tricuspid regurgitation velocity of 4 m/s predicting elevated RV systolic pressure (64 mm Hg plus RA pressure). LA, left atrium; LV, left ventricle.
Angling the transducer anteriorly from the four-chamber view brings the left ventricular outflow tract into view; further anterior angulation (or para-apical) provides another excellent view of the pulmonary valve and RVOT. Doppler determination of any important RVOT obstruction is important with respect to planning therapeutic catheterization or surgery.
Suprasternal Notch Views
The right and left branch pulmonary arteries are seen well from suprasternal notch imaging. In a short-axis or coronal plane, the LA can be visualized inferior to the right pulmonary artery. Sweeping the transducer from left to right should demonstrate all four pulmonary veins connecting to the LA (the “crab” view). Rotating the transducer 90 degrees to the long-axis plane is helpful to rule out an anomalous left upper pulmonary vein to a vertical vein. From this imaging plane, color Doppler flow coursing superiorly toward the transducer, entering the innominate vein, should alert the echocardiographer to the possibility of an anomalous left pulmonary vein.
Additional Imaging
High right parasternal imaging can provide an additional window to evaluate the SVC-RA junction and superior portion of the atrial septum. In some patients, this imaging plane may provide a “bi-caval” view and further assessment of the superior and inferior septal rims of a secundum defect optimally visualizes a high sinus venosus ASD.
In the patient with right-sided cardiac enlargement on transthoracic imaging but no identifiable ASD or abnormality of pulmonary venous return, a transesophageal echocardiogram (TEE) is indicated. TEE imaging of the atrial septum is usually of sufficient quality to demonstrate most defects; however, one must be attentive to the very inferior/posterior portion of the atrial septum, as proximity to the imaging probe may obscure visualization. Cardiac MRI may also be of benefit if the atrial septum is adequately visualized from TEE but the pulmonary venous connections cannot be resolved.
TWO-DIMENSIONAL ECHO ANATOMY AND IMAGING
Patent Foramen Ovale
A PFO is located immediately beneath the superior limbus and is typically a small defect. In the majority of neonates undergoing echocardiography, a small amount of left-to-right shunting is present through the PFO with color Doppler echocardiography. Spontaneous closure occurs in the majority of patients, although probe patency persists in approximately 25%. In the older patient with clinical evidence of paradoxical embolism, a small defect or larger redundant septal flap, along with a tunnel, may be seen. In such patients, TEE will usually be needed to document septal anatomy in detail, with injection of agitated saline through an intravenous line to confirm a right-to-left shunt if not seen on baseline color Doppler interrogation.
Secundum Atrial Septal Defect
A secundum ASD produces characteristic dropout in the central-most portion of the atrial septum. Most defects are relatively elliptical in shape. Assessment from multiple views is needed to fully evaluate the maximum ASD dimensions, septal rims, and relationship of the ASD to surrounding cardiac structures. With the widespread availability of transcatheter device closure of secundum ASD, the echocardiographer plays a critical role in assessment and patient selection for catheter-based therapy. One must also exclude the presence of fenestrations or aneurysmal septal tissue in association with a secundum ASD, although these often remain amenable to device closure. It is very important to rule out any defects that would require surgical attention (i.e., anomalies of pulmonary veins).
A typical secundum ASD is seen in the subcostal (coronal) four-chamber orientation (Fig. 6.7) and in the short-axis (sagittal) imaging plane as a central area of septal dropout within the atrial septum with surrounding rims of tissue present. From the subcostal four-chamber imaging plane, a rim of tissue should separate the SVC entrance to the RA and the right upper pulmonary venous entrance to the LA from the ASD. More inferiorly with leftward and posterior angulation toward the crux of the heart, there is typically a larger rim of tissue separating the atrial defect from the atrioventricular valves and CS. Angling anteriorly, the defect can be seen posterior to the aorta; however, the retroaortic rim of tissue will be seen more definitively in the parasternal short-axis view. Subcostal short-axis imaging shows the superior/anterior rim of the defect just beneath the SVC–RA junction. The normally connected right upper pulmonary vein is seen entering the LA posterior to this rim. One of the rims that may be most difficult to visualize adequately in patients with secundum ASD is the posterior/inferior rim. Parasternal short-axis images also often demonstrate this rim posteriorly. Subcostal short-axis imaging is often diagnostic when image quality is adequate (see Fig. 6.7C). It is important to remember that the Eustachian valve tissue, which may be prominent and often confused with an ASD septal rim, enters the RA anterior to the inferior vena cava (for an example of a complex ASD see Fig. 6.8).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


