Pulmonary embolism (PE) as a complication of deep venous thrombosis of the lower extremity (and rarely of the upper extremity) is a widely recognized clinical problem causing an estimated 240,000 deaths per year in the United States alone (1). Caval filtration is designed to prevent clinically significant PE in high-risk patients. The use of inferior vena caval (IVC) filters has seen a dramatic increase in the past several decades with nearly 140,000 filters placed worldwide in 2003 (2).
Despite widespread use and acceptance, there remain little prospective data on long-term outcome of filters. Knowledge of anatomy, indications for placement, and techniques for removal are essential for optimal management of patients.
HISTORY OF CAVAL INTERRUPTION
Caval interruption was first suggested by Trousseau in 1868 (3–5). The first IVC ligation was performed in the 1940s. This was followed by IVC compartmentalization with sutures, staples, and serrated clips (1,4). These techniques were complicated by high rates of caval thrombosis, clinically significant lower extremity swelling, recurrent PE, and significant mortality (1,4). The first surgically implanted endovascular filter was the Mobin-Uddin umbrella filter in 1967, which suffered from high rates of IVC thrombosis (1,6). The next major advance in IVC filtration was the design of the Greenfield filter in 1973 (1,4,7). This filter had a conical design and a high packing efficiency allowing 70% to 80% of the filter to be filled with clot without inducing a significant pressure gradient or altering blood flow across the filter. Initially placed surgically, the Greenfield filter became the first standard in IVC filter technology. The first percutaneous Greenfield IVC filter was placed in 1984 (1,8). The past several decades have shown an explosion in filter technology, design, and usage.
ANATOMY
Normal Anatomy
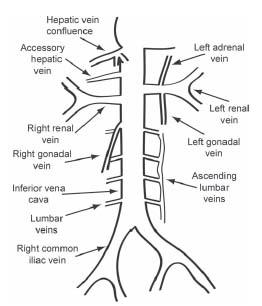
The IVC is formed by the confluence of the common iliac veins at the L5 vertebral level (Fig. 29.1). The right common iliac vein is shorter and more vertical. The right common iliac artery passes over the left common iliac vein and may cause physiologically important obstruction (Fig. 29.2). The IVC ascends in the retroperitoneal space adjacent to the spine to the right of the aorta. It receives major drainage from iliac, lumbar, gonadal, renal, and hepatic veins (9).
Four pairs of lumbar veins collect blood from the abdominal wall and posterior musculature and drain into the IVC (9). They are joined by vertically oriented ascending lumbar veins that anastamose with paravertebral veins more centrally (Fig. 29.3). Ascending lumbar and paravertebral veins are important sources of collateral flow in IVC obstruction. Above L2, the ascending lumbar veins continue to become the azygos vein to the right of spine and the hemiazygos vein to the left of spine (9). The hemiazygos vein typically drains into the azygos vein at approximately T8, which then drains into the superior venal cava (SVC) at the level of the aortic arch (9) (Fig. 29.4).
The renal veins enter at the L1/2 level (Figs. 29.5 and 29.6). The right renal vein is typically slightly lower than the left. Several clinically important variations of renal vein anatomy are common and will be discussed below. The right gonadal vein drains directly into the IVC and its course parallels the IVC (9). The left gonadal vein typically drains into the left renal vein.
The hepatic veins enter immediately inferior to the diaphragm on the right anterior aspect of the IVC (Fig. 29.7). The IVC then drains into posterior aspect of the right atrium. Accessory hepatic veins are common and enter the right side of the IVC between the renal veins and main hepatic venous confluence (Fig. 29.8). Phrenic veins and adrenal veins are typically small and are not usually clinically significant to filter placement.
Variant Anatomy
Congenital anomalies of renal veins occur in up to 30% of individuals (10–12). They are clinically relevant to caval filtration, as IVC filters should be placed below the level of the lowest renal vein to prevent thrombus bypassing the filter through major collateral pathways. Multiple right renal veins, circumaortic left renal vein (Fig. 29.9), and retroaortic left renal vein are the most common variants (Fig. 29.10) (11,13).
Congenital anomalies of the IVC are less common with an incidence of 2% to 3% (11,13). They are also clinically relevant to placement of IVC filters and result from the complex embryogenesis of IVC development (13). The most common variations are duplicated IVC, left IVC, and interrupted IVC with azygos/hemiazygos continuation (13). A duplicated IVC is present when each common iliac vein drains into its own vena cava, with the left IVC typically draining into the left renal vein. The left renal vein then crosses anterior to the aorta and joins the right IVC to form a normally oriented suprarenal IVC. In patients with a left-sided (transposed) IVC, the infrarenal cava is located to the left of the aorta (Fig. 29.11). The left-sided cava typically drains into the left renal vein, which then crosses anterior to the aorta and joins with the right renal vein forming a normally oriented suprarenal IVC. Azygos continuation of the IVC is present when the IVC is interrupted between the hepatic and renal veins. Drainage of the infrarenal IVC is through the azygos/hemiazygos systems. The hepatic segment forms an IVC stump and drains into the right atrium. This anomaly is associated with congenital heart disease and polyspenia/asplenia syndromes. Congenital absence of the IVC is rare and is present when there is no infrarenal IVC and all abdominal venous drainage is via the ascending lumbar and paravertebral veins (Fig. 29.12).
Figure 29.1 • Normal anatomy of the inferior vena cava (IVC).

Figure 29.2 • Physiologic compression of left common iliac vein (CIV) by right common iliac artery (CIA). A: IVC venogram demonstrating the physiologic compression (black arrow) of the right CIA on the left CIV. If this causes deep venous thrombosis of left lower extremity, it is known as May–Thurner syndrome. B: Axial CT demonstrating physiologic compression of the right CIA (white arrow) on the left CIV (black arrow).

Figure 29.3 • IVC venogram demonstrating lumbar (white arrow) and ascending lumbar (black arrow) veins.
Figure 29.4 • Normal anatomy of azygos/hemiazygos system.

Figure 29.5 • A typical IVC venogram with streaming inflow from renal veins (arrows). Note the marker pigtail catheter for IVC sizing.
INDICATIONS FOR IVC FILTER PLACEMENT
IVC filtration is designed to prevent clinically significant PE by trapping venous clot before it reaches the lungs. It does nothing to treat or prevent venous thrombosis and is not a substitute for therapeutic anti-coagulation, which remains the standard medical therapy for venous thromboembolism. For optimal treatment of thromboembolic disease, therapeutic anti-coagulation should be resumed as soon as possible (14,15).
Caval filters are used for two primary indications: (1) the prevention of PE in patients with known deep venous thrombosis (DVT) or pulmonary embolism where standard medical therapy is contraindicated, ineffective, or has to be interrupted and (2) for prophylaxis in patients without known venous thromboembolism when the risk for the development of DVT/PE is high and medical therapy is contraindicated. Table 29.1 lists common indications for filter placement (1,4,15,16).
Figure 29.6 • IVC venogram demonstrating many aspects of normal anatomy not typically seen. The right renal vein (long black arrow) is lower than the left and is nearly fully opacified. The left renal vein (long white arrow) appears as streaming inflow as do the hepatic veins (short black arrow). A lumbar vein (white arrowhead) connects to the left ascending lumbar vein (short white arrow).

Figure 29.7 • Normal IVC venogram with opacification of the hepatic venous confluence (black arrow).

Figure 29.8 • IVC venogram demonstrating a typical appearance of an accessory right hepatic vein (black arrow). Normal renal veins are demonstrated with white arrows.
CONTRAINDICATIONS
The only absolute contraindication to filter placement is an inability to place a filter due to IVC thrombosis or thrombosis of all available access vessels. Patients with an uncorrectable coagulopathy should be treated with caution, although control of access site bleeding is typically not problematic. A temporary central venous catheter may be left in place to tamponade bleeding at the insertion site as an alternative in critically ill patients who may benefit from additional access. Sepsis is no longer considered to be a contraindication to filter placement (1,16,17).
LOCATION OF FILTER PLACEMENT
Infrarenal IVC Filter Placement
The most common placement for an IVC filter is in the infrarenal vena cava. Ideal placement is with the filter apex at the level of the lowest renal vein (Fig. 29.13). Placement at this level prevents clot from bypassing the filter through circumaortic or accessory renal veins. Placement of the filter apex at or near the lowest renal vein reduces the dead space above the filter. If the filter is placed low in the IVC, clot may form in the IVC between the level of the filter and the lowest renal vein, which may embolize unprotected to the lungs.
Figure 29.9 • Left circumaortic renal vein. A: IVC venogram demonstrating a circumaortic left renal vein (black arrow). The left renal vein draining the superior segment of the left kidney is indicated with a white arrow. B: Oblique CT maximum intensity projection (MIP) demonstrating a left circumaortic renal vein (different patient).

Figure 29.10 • Left retroaortic renal vein. A: IVC venogram demonstrating abnormally low insertion of a left renal vein (black arrow). This is a typical appearance of a retroaortic left renal vein. The right renal vein is indicated with a white arrow. B: Axial CT correlate demonstrating the appearance of a retroaortic left renal vein (black arrows). Note the low insertion onto the cava (axial level at the inferior pole of the kidneys).
Figure 29.11 • Left-sided IVC. A: IVC venogram demonstrating a typical appearance of left-sided IVC (long black arrow) that joins the left renal vein (white arrow). Right renal venous inflow is also demonstrated (short black arrow). B: Curved CT multiplanar reconstruction (MPR) demonstrating a patient with a left-sided IVC and a filter (white arrow) in place.
Suprarenal Filter Placement
Occasionally, placement of an IVC filter above the renal veins is necessary (Fig. 29.14). The main indications for placement of a filter in the suprarenal location are listed in Table 29.2. Suprarenal filters perform similarly to infrarenal filters in terms of effectiveness and complications based on limited data (18,19). If suprarenal filter placement is required, a Bird’s Nest filter should be avoided as the wires may prolapse into the heart. Classic teaching is to place a suprarenal filter in pregnant patients to avoid the effects of uterine compression on the infrarenal IVC, which may make penetration of the IVC wall by the filter legs more likely with possible injury to the uterus, although there is little evidence to support this (18,19). When placing an IVC filter during pregnancy, a jugular approach with pelvic shielding is preferred to minimize radiation dose to the fetus.
SVC Filter Placement
Occasionally, upper extremity thrombosis and clinically significant PE will necessitate placement of an SVC filter. While limited literature suggests that SVC filter placement is safe and effective, it should be performed only in carefully selected patients with appropriate precautions (4,20,21).
Anatomic considerations for SVC filter placement include a short overall length of the SVC (approximately 7 cm), inflow from the azygos vein, and close proximity to the heart and great vessels, which induces cardiac motion (20). Pericardial tamponade is an additional risk of SVC filter placement secondary to filter leg perforation into the superior pericardial reflection, which is in close association with the SVC (20,21). Males younger than 60 years are felt to be at higher risk, for unclear reasons (21). Placement of the filter apex into the right atrium should be avoided to prevent arrhythmias and cardiac wall injury. The risk of prolapse of the wires from a Bird’s Nest filter (Cook) into the right atrium makes this filter a less optimal choice for this location.
Existing central venous catheters must be removed or retracted out of the deployment area during placement. High-quality venography, careful sizing, and accurate placement are required to prevent complications. Most of the available data for SVC filtration are with permanent filters, although the use of retrievable filters may be considered, particularly when the anticipated duration of filtration is considered to be short. There is a paucity of data regarding the safety of retrieval.
Figure 29.12 • Congenital absence of IVC. In this patient with congenital absence of the IVC, all venous drainage is through the hypertrophied ascending lumbar veins (black arrow) and more central paravertebral veins (white arrow). IVC obstruction may have a similar appearance.
Adequate documentation of SVC filter placement in the medical record is advisable, as these filters may be displaced by attempts at central line placement. Subsequent lines should ideally be placed under fluoroscopic guidance.
DEVICES
Filter Types
Filters can be classified into three categories: permanent, retrievable (optional), and temporary (Table 29.3; Figs. 29.15 and 29.16). Permanent filters cannot be repositioned or removed and are left in place as lifelong devices. There are many available permanent filters with a variety of designs and delivery systems.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree