Zoonotic and Other Unusual Bacterial Pneumonias
INTRODUCTION
Many different microorganisms can infect the lung parenchyma; this chapter focuses on zoonoses, human commensals, and other unusual bacterial pathogens. Routes of spread to the lungs are few, clinical presentations overlap, radiologic appearance often is nonspecific, and pathophysiologic mechanisms are limited. Making a clinical diagnosis of pneumonia is relatively easy; defining the causative agent can be difficult. The search for the specific etiologic agent is driven by the desire to administer a specific antimicrobial drug as soon as possible hopefully to avoid progressive functional impairment and to contain spread to other individuals or to a community. Identifying a pathogen may, in turn, expand epidemiologic considerations and public health awareness.
In the almost four decades that have passed since the epidemic of acute respiratory disease due to Legionella erupted among delegates to the American Legion Convention in Philadelphia in 1976, physicians, microbiologists, and epidemiologists have become increasingly prepared for the diagnostic challenges of patients with unusual pneumonias. More recent examples of this multidisciplinary approach include the rapid diagnosis of Q fever (due to Coxiella burnetii), early identification of cases of Chlamydia pneumoniae and Chlamydia psittaci, and the etiology of acute respiratory infections due to obscure viral agents like Hantavirus pulmonary syndrome and the newer Coronavirus respiratory illnesses including severe acute respiratory syndrome (SARS). SARS was an excellent example of a zoonotic infection with an initiating event and subsequent person-to-person spread. Similarly, Middle East respiratory syndrome coronavirus (MERS) has been linked to dromedary camel exposure, with subsequent inter-human spread. The complexity of the definition of an etiologic agent in the diagnosis of pneumonia of obscure origin is highlighted in many of the examples mentioned here. Most are discussed in detail elsewhere in this text; the focus will be exclusively on bacterial pathogens most often associated with animals or animal products.
Zoonotic infections can reflect an initial event, followed by person-to-person secondary cases, typical of viral diseases like SARS coronavirus, and occasionally observed with Yersinia pestis. While the avian influenza virus (H5N1) has primarily spread to individuals in proximity to chickens, the novel H1N1 influenza in 2009 to 2010 demonstrated an anthropocentric cycle that led to a worldwide pandemic, despite being of zoonotic origin. New zoonoses are continually emerging: A recent outbreak in a monkey colony of deadly titi monkey adenovirus, not known to infect humans, was shown to infect a researcher and a family member, without further spread.1 Similarly, Streptococcus equi subsp. zooepidemicus (Streptococcus zooepidemicus)2 has recently been shown to cause pneumonia in humans after exposure to horses, dogs, and unpasteurized milk.
Companion animals have been the etiology of numerous zoonotic pneumonias, including Pasteurella multocida,3,4 Chlamydia psittaci,5 Rhodococcus, and Bordetella bronchiseptica (especially in immunocompromised hosts6–9). In a study in Spain, contact with pets was associated with an increased risk for community-acquired pneumonia, which tended to be higher as the number of pets increased; the relative risk was similar for birds, cats, and dog exposure.10 There are multiple examples of animals serving as reservoirs of antibiotic-resistant bacteria, such as methicillin-resistant Staphylococcus aureus (MRSA) (companion animals,11,12 pigs13), some in association with nosocomial ventilator-associated pneumonia,11 and highly resistant Enterobacteriaceae.14 Nonetheless, experts usually permit companion animals even for immunocompromised hosts, balancing the risk of infection with perceived benefit.6,15 Wild and farm animals may also harbor zoonotic pneumonia pathogens. P. multocida has been associated with pneumonia in bighorn sheep,16 lambs,17 and pigs.18 Y. pestis has been associated with numerous wild mammals; recent work suggested a correlation between seropositivity in coyotes and human disease.19
Many of the zoonotic bacteria discussed in this chapter are recognized as pathogens that can be weaponized for use by terrorist organizations. The global community is developing rapid diagnostics and countermeasures to warn, protect, and treat people exposed to a biologic weapon. Unfortunately, we have the experience of the “mailroom” anthrax event of September 2001 to illustrate the potential of an inhalational disease causing illness, casualties, public panic, diagnostic and therapeutic decisions, as well as the expenses of decontamination and the challenges of criminal investigations.20 Physicians need to become familiar with the typical and atypical spectrum of disease caused by these pathogens, including the impact and variable expression of a large inoculum aerosol release in a crowded urban area or building.
This chapter focusses on the epidemiologic and clinical features that should be helpful in evaluating typical and atypical features of pneumonia (Table 138-1). The unique properties of these bacteria exemplify how bacteriology, molecular biology, ecology, epidemiology, and pathogenesis serve as clues to earlier etiologic diagnosis and specifically targeted therapies (Table 138-2). The movements of immigrant populations, adventure and other forms of travel, the immune status of individuals, the latency of pathogens, and the legal and illegal transportation of live animals and animal products contribute to the importance of zoonotic pneumonias. Ultimately, the diagnosis is heavily dependent on a detailed history along with appropriate diagnostic studies.
TABLE 138-1 An Overview of Zoonotic and Other Unusual Pneumonias
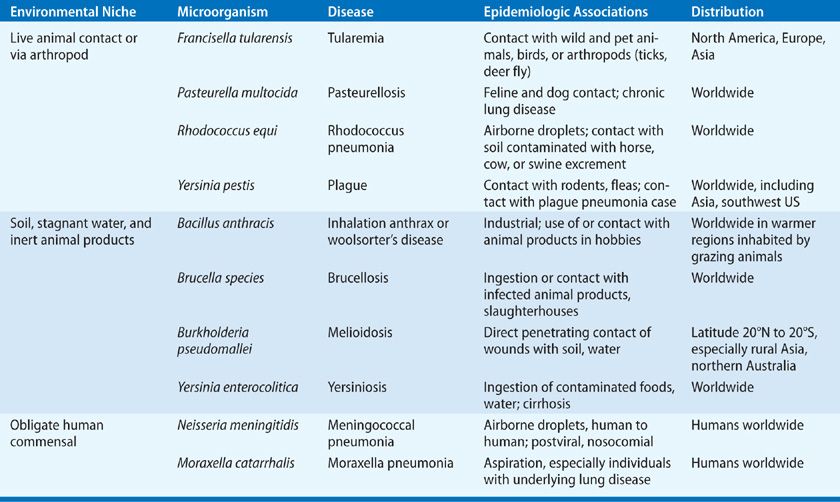
TABLE 138-2 Diagnostic Studies and Treatment Recommendations in Zoonotic Pneumonias
ZOONOTIC BACTERIAL PNEUMONIAS
This section focuses on diseases that are spread to humans predominantly from animal contact, including pasteurellosis, the plague, tularemia, and Rhodococcus. The diagnosis usually depends on a careful animal exposure history. Once alerted, the clinician can request appropriate studies, as these diseases may be more elusive to diagnose.
 PASTEURELLA MULTOCIDA
PASTEURELLA MULTOCIDA
P. multocida is a common commensal of the oral cavity of most felines and many dogs and a frequent respiratory pathogen in animals and birds.3 The respiratory tract is the second most common site of Pasteurella infection following skin and supporting tissues. Respiratory infections are probably underreported. Elderly people with structural lung diseases such as emphysema, bronchiectasis, or malignancy are at higher risk for pulmonary infection. Immunocompromised patients, including those with AIDS, may be prone to spontaneous infection without close contact or traumatic dog or cat exposures.
Bacteriology
P. multocida is a small gram-negative bipolar-staining coccobacillary organism that resembles Haemophilus spp. and may form pairs and chains. Rapid growth on blood agar and inhibition by MacConkey medium separate this microorganism from other common components of the respiratory flora, including Haemophilus spp. Numerous virulence factors in P. multocida have been identified. These include the capsule in serogroups A and B (which interferes with phagocytosis), a toxin in strains causing atrophic rhinitis in pigs, lipopolysaccharide endotoxin, several iron acquisition proteins (e.g., TonB, ExbD, and ExbB), and the putative filamentous hemagglutinins PfhB1 and PfhB2.
Ecology, Epidemiology, and Pathogenesis
In cats and other felines, the organism resides periodontally in the anterior regions of the mouth. Isolates from dogs are characteristically from the posterior pharynx. Many birds and domestic and wild animals worldwide harbor this organism as a commensal in oral or gastrointestinal areas. P. multocida is occasionally found in the secretions of persons with chronic lung disease, especially those with bronchiectasis and domestic animal contacts. A recent report links the domestic cooking and ingestion of pig trotters (feet) with subsequent pneumonia.21 Human-to-human transmission has been documented from mother to newborn infant, resulting in neonatal pneumonia. The organism can survive in soil and water for more than 3 weeks and in animal carcasses for approximately 2 months. In about half of the cases of respiratory disease, no clue to airborne spread exists, but there are usually cats in the local environment.
Pathogenic strains have a polysaccharide capsule that inhibits phagocytosis, and they contain endotoxin in the cell envelope. Exotoxins and other pathogenicity-promoting factors have not been specifically identified (see Bacteriology). Almost all patients who develop respiratory infections have underlying chronic pulmonary disease. Aspiration probably initiates active infection. Necrosis and lung abscess, empyema, septicemia, and transbronchial spread to other lung segments have been described.
Clinical and Radiologic Features
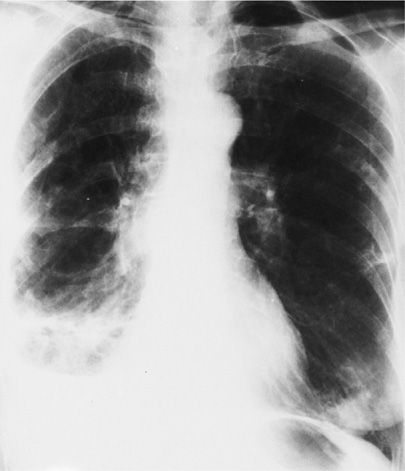
The clinical features of P. multocida respiratory disease include worsening of the patient’s baseline pulmonary function—especially when high fever, tenacious secretions, and pleural effusions develop. Radiologic changes include lobar, multilobar, or diffuse patchy infiltrates, usually sparing the upper lobes, superimposed on underlying chronic lung disease (Fig. 138-1). Effusions, including empyema, have been noted in approximately 20% of cases. Bacteremia has been reported in up to 55% of patients with pneumonia, and endocarditis has occasionally complicated bloodstream invasion.3
Figure 138-1 Bilateral pneumonia due to Pasteurella multocida in a 69-year-old woman suffering from chronic obstructive pulmonary disease and a prior right lower lobectomy for carcinoma. Infiltrates disappeared with penicillin therapy.
Diagnosis and Differential Diagnosis
Diagnosis depends on isolation of the organism from sputum, pleural fluid, or blood. Usually the pathogen can be identified with the routine methods of the diagnostic laboratory. The bipolar gram-negative staining bacilli resemble Brucella spp., Y. pestis, Francisella tularensis, Burkholderia (formerly Pseudomonas) pseudomallei, and Haemophilus spp., but the clinical history of exposure to cats and/or dogs and bacteriologic characteristics can rapidly clarify the identification.
Treatment
Most strains are exquisitely susceptible to penicillin or ampicillin. The third-generation cephalosporins, cefotaxime and ceftriaxone, are as active as penicillin and more potent than earlier-generation relatives. Oral preparations of cephalosporins and penicillins are not recommended for treating pneumonias caused by P. multocida due to reduced bioavailability. The newer fluoroquinolones, including moxifloxacin and levofloxacin, are very active in vitro against P. multocida and other Pasteurella spp., although there is limited published experience in humans. Chloramphenicol, with consideration of potential toxicities, and tetracycline are effective alternatives when a history of allergic reactions precludes use of a β-lactam agent.
 YERSINIA PESTIS
YERSINIA PESTIS
This organism left an indelible mark on humanity long before its late 19th-century isolation and characterization. The cause of three major pandemics from the 6th through the 19th centuries, pulmonary disease in a few victims led to aerosol spread to countless others, resulting in acute primary pneumonia and the “black death” of epidemic plague. During the period from 1932 through the end of World War II (1945) the Japanese biologic warfare program included the use of aerosol drops of Y. pestis as well as infected flea disseminations in occupied China and at the infamous experimental Unit 731. The effectiveness of the flea drops remains in question to this day but the aerosol dispersal definitely led to clinical pneumonic plague. A contemporary global scare emanated from India’s Maharashtra state in 1994, with reports of suspected pneumonic and bubonic plague; interestingly, some suspected cases were subsequently found to be due to B. pseudomallei.22 In the United States, fewer than 20 cases of plague are reported yearly, of which one in five has lung involvement but without any secondary cases. Early recognition and specific therapy, combined with isolation procedures and appropriately directed prophylaxis of contacts should help maintain the record of no human-to-human transmission in the United States since the 1920s. When plague is diagnosed outside an endemic area, bioterrorism should be considered. When Yersinia pneumonia occurred in a couple who recently became ill in New York City, the event was originally investigated as a potential bioterrorism event until their travel from an endemic area of plague (Santa Fe County, New Mexico) was discovered.23 Although the bacteria are difficult to process, handle, and disperse in an aerosol form, person-to-person airborne spread is very efficient and laboratory accidents have occurred.
Bacteriology
Y. pestis is a bipolar-staining, gram-negative bacillus closely related to Escherichia coli and other Enterobacteriaceae. It grows well on blood or MacConkey agar and is identified definitively with differential biochemical tests, agglutination reactions, and direct fluorescent antibody staining. Concerns for laboratory safety have led to routine of Biosafety level 2 (BSL-2) laboratory procedures as well as better communication between clinicians and microbiology laboratory personnel in suspected cases.
Ecology, Epidemiology, and Pathogenesis
In the United States, Y. pestis is endemic in rock squirrels, prairie dogs, rabbits, rats, and other small ground animals primarily in the Southwest. Spread among animals occurs via several species of rodent fleas, especially Xenopsylla cheopis. Domestic animals that wander outdoors, like cats, can become infected by direct contact with sick rodents or via rodent flea bites. In addition, cats and dogs can inadvertently carry fleas to the home. Occasionally rodent die-offs, called epizootics, occur, and many dead animals can be found with viable organisms in carcasses and in the soil surrounding ground dwellings.
In the United States, spread to humans occurs when an infected flea feeds on a susceptible person. Living or working in proximity to local enzootic “hot spots” places certain groups (such as Native Americans, geologists, hikers, veterinarians, and pet owners) at risk. Bubonic and cutaneous plague are usually acquired on exposed areas by contact with infected fleas, but aerosols from ill animals or carcasses can lead to primary pneumonia, pharyngitis, or conjunctivitis. Several cases of cat-to-human aerosol spread have been associated with respiratory infection or submandibular abscesses in pets. A great concern of physicians caring for patients with respiratory infection is the potential for rapid airborne dissemination, especially during coughing and face-to-face contacts, resulting in primary pneumonia and the rapid development of acute respiratory distress syndrome (ARDS).
After the organism gains access to human tissues at 37°C, rapid multiplication occurs. The polysaccharide capsule imparts virulence properties that include resisting phagocytosis and persistence of bacteria within nonsensitized monocytes. Virulence factors include a potent endotoxin and V and W antigens of the cell envelope, which influence intracellular survival. Recent work with a mouse model has demonstrated a biphasic illness with an initial anti-inflammatory phase that rapidly progresses to a highly proinflammatory state by 48 hours and death by 3 days. Microarray analysis demonstrates a change in the expression of about 10% of the bacterium’s genes after it infects a host.
Clinical and Radiologic Features
Clinical presentations are highly varied and include subclinical cases (positive serology without evidence of disease), a chancriform skin lesion (pestis minor abortive bubonic plague), pharyngitis, bubonic plague, septicemic plague, pneumonic plague, and meningitis. The clinical presentation of pneumonia depends on the mechanism of spread. In contemporary experience in the United States, cases have all been secondary to bubonic plague, primary septicemia without an overt skin lesion, or inhalation of droplets from an infected pet cat. The onset of respiratory disease follows after days to a week of a febrile illness, and is ushered in by the gradual onset of cough, dyspnea, and increasing toxicity. Pink to hemorrhagic frothy sputum, pleurisy, and respiratory distress are additional symptoms. The unique feature in most cases of pneumonia is the epidemiologic association with classic bubonic plague; that is, in a person in or from an endemic area or who has had contact with a pet cat ill with respiratory symptoms or a facial abscess. From histories of primary inhalation pneumonia described previously, exposure to an index case may be followed by the rapid development of a fulminating respiratory illness, with dyspnea, cyanosis, and thin, watery sputum that rapidly becomes hemorrhagic. The clinical picture is not unlike that of overwhelming pneumococcal pneumonia, with marked toxicity and mental torpor associated with progressive cyanosis.
The radiologic features of secondary pneumonia include basal segment nodular to hazy air space infiltrates, hilar and mediastinal node hypertrophy, and occasionally pleural effusions.24 In primary pneumonia, infiltrates may be minimal during the first 24 hours, followed by progressive air space disease resembling ARDS or pulmonary edema.
Diagnosis and Differential Diagnosis
The presence of characteristic bipolar-staining gram-negative bacilli in sputum suggests the diagnosis when epidemiologic clues are present. Cultures of blood, sputum, and lymph node aspirates often yield positive results. Direct fluorescent antibody staining can provide immediate etiologic confirmation. A passive hemagglutination test can be performed as a confirmatory study on acute and convalescent sera at selected reference laboratories such as CDC. Rapid assays for diagnosis of plague are under active development, especially given the interest in its potential as a bioterror agent. Other acute respiratory infections caused by microorganisms that appear as gram-negative bacilli with bipolar staining must be considered, including F. tularensis and P. multocida.
Treatment and Prevention
The combination of streptomycin and tetracycline has been the treatment of choice for serious plague infections. Gentamicin can be substituted for streptomycin if intravenous therapy is necessary or streptomycin is not available. Doxycycline and fluoroquinolones such as ciprofloxacin may also be effective in treating pneumonia; chloramphenicol is a potential option, noting toxicities. Multidrug-resistant plague has been reported and clinicians should be aware of local trends as well as the potential for terrorist-instigated modifications in antibiotic susceptibility.
Persons suspected of having plague pneumonia should be rapidly isolated, and strict contact, respiratory, and conjunctival precautions instituted. Anyone exposed face to face with a coughing patient, including healthcare workers, should be given preventive tetracycline or ciprofloxacin (trimethoprim/sulfamethoxazole is used in pregnant women and children). Isolation procedures are continued until productive cough is no longer present or sputum cultures are negative for Y. pestis.
A vaccine is available for laboratory workers and others with frequent exposure to the microorganisms or hyperendemic areas, although it does not appear to protect against pneumonic plague. Careful surveillance of ground rodent populations, posting warnings in endemic regions, watching for die-offs that indicate epizootic spread, and spraying for local flea control may also be effective preventive measures along with public education.
 FRANCISELLA TULARENSIS
FRANCISELLA TULARENSIS
Tularemia is a common animal disease in the United States as well as many other regions of the temperate globe. The causative agent, F. tularensis, is ubiquitous, distributed among many species of wild and domestic animals and birds. As with plague, the major clinical manifestations include skin lesions and swollen or draining regional lymph nodes. In addition to primary inhalation pneumonia, pulmonary invasion is seen following bacteremia in 10% to 15% of ulceroglandular cases and in more than 50% of patients with the typhoidal syndrome. Approximately 150 human cases are reported in the United States yearly, but this is probably an underestimate; approximately 40% of all tularemia cases each year occur in Arkansas, Oklahoma, and Missouri.25 In the summer of 2000, an outbreak of pneumonic tularemia occurred on Martha’s Vineyard, Massachusetts, and 11 of 15 patients were diagnosed as primary pneumonic tularemia; lawn mowing and brush cutting were found to be risk factors for infection.26 An epidemic of tularemic pharyngitis from ingestion of contaminated well water and food occurred in war-torn Western Kosovo primarily among young children originally suspected of having group A Streptococcal pharyngitis, with no secondary pneumonias reported.27
Bacteriology
F. tularensis is a fragile-appearing gram-negative coccobacillary organism that is quite fastidious and grows poorly on artificial media unless fortified with serum and cysteine (or sulfhydryl compounds). The potential for laboratory-acquired inhalation or ingestion-associated disease is great. Most routine laboratories will not attempt to culture the organism, leaving this to special reference centers. Identification is on the basis of morphologic and biochemical determinants, but direct fluorescent staining or agglutination reactions with specific antisera are also useful.
Ecology, Epidemiology, and Pathogenesis
The organism is associated with more than 100 species of wild and domestic animals and birds, including aquatic mammals, but most clinical cases arise from contact with rabbits, squirrels, or arthropods. Of great concern is the recent fad for exotic pets; tularemia in prairie dogs has led to spread in holding pens and to human transmission. In cold weather the organism can persist in water and mud environments for weeks to months. Bloodsucking arthropods, especially ticks and deerflies, act as reservoirs capable of harboring the pathogen for long periods and are responsible for dissemination among wildlife species as well as infecting people. Domestic cats represent a potentially increasing problem.28
Less than half of human cases are acquired from contact with infected animals during hunting, trapping, and other outdoor pursuits, especially during colder months. In southern areas, or in the summer season in northern latitudes, gardening and lawn mowing without animal contact are becoming important methods of dissemination from disturbed soil. Bloodsucking arthropods constitute a significant and increasing mode of spread, especially in warmer seasons. Ingestion of contaminated food or water,27 animal bites, conjunctival contact, and aerosol dissemination are also important mechanisms for acquiring the pathogen. In recent years, cases secondary to arthropods have been more frequent than those associated with direct animal contact, although domestic cat bites28 and airborne spread appear to be increasing. Human-to-human transmission is rare, in contrast to the significant theoretical potential for spread of pneumonic plague. If F. tularensis were to be used by terrorists, aerosol release would most likely be the mode of spread, with respiratory as well as other locations (skin, conjunctival, pharynx) occurring in the exposed population. Those criminally processing this organism would be at great risk for laboratory infection.
F. tularensis contains a number of protein and polysaccharide antigens in the cell envelope as well as an endotoxin component that is similar to endotoxins of other gram-negative microorganisms. Very little is known about other mechanisms of pathogenesis. The organism is capable of remaining viable within the reticuloendothelial cells of nonimmune subjects and in macrophages that have not been activated by recent exposure to other intracellular pathogens. As few as 10 to 50 organisms can initiate disease following cutaneous penetration or by inhalation, but a significant number are required when the challenge is through ingestion of contaminated water or foods. Local growth usually is followed by regional node suppuration and occasionally bacteremic dissemination to many organs, including the lungs. Primary pneumonia follows inhalation of organisms, resulting in numerous areas of inflammation, necrosis, a tendency to granuloma formation, hilar and mediastinal adenopathy, and pleural inflammation and effusion.
Clinical and Radiologic Features
Respiratory disease is heralded by the onset of a nonproductive cough, usually in a febrile patient ill with the ulceroglandular form of tularemia.29 In the absence of a local chancriform lesion or a tender swollen lymph node (bubo), the disease may be dominated by constitutional symptoms, with high fever, severe headache, prominent myalgias, and shaking chills (typhoidal tularemia). Pneumonia following an inhalation exposure results in cough, dyspnea, and occasionally pleurisy. Respiratory disease can be subtle, and the diagnosis may be apparent only if a chest radiograph is done. Pleural effusions may be serosanguineous or frankly bloody, an uncommon finding with other pulmonary infections except anthrax.
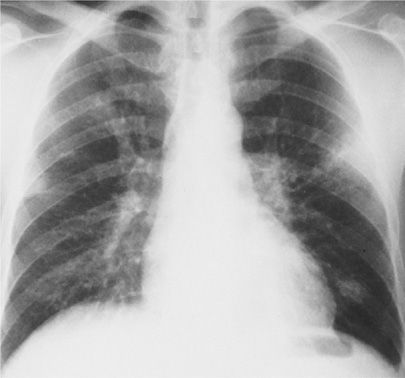
Radiologic changes include evidence of parenchymal and pleural diseases, which is often out of proportion to the findings on examination. Diffuse areas of bronchopneumonia occur, with hilar node enlargement and occasionally mediastinal widening, similar to radiologic appearance of anthrax. Unilateral or bilateral pleural effusions are often noted (Fig. 138-2).
Figure 138-2 Patchy nodular and bronchopneumonia, hilar adenopathy, and left pleural effusion due to Francisella tularensis in a 38-year-old veterinarian exposed to a cat dying with a respiratory infection. All the findings resolved with tetracycline therapy.
Diagnosis and Differential Diagnosis
Any febrile patient with animal, arthropod, or landscaping exposure in an endemic region, especially presenting with a chancriforme skin lesion and/or a tender lymph node should be evaluated for tularemia. Cough, when present, is usually nonproductive, and blood cultures are seldom positive. Characteristic organisms are rarely seen in pleural fluid or aspirates of suppurating nodes. Direct fluorescent antibody staining of exudates can confirm the diagnosis, but this method is not widely available. Serologic testing remains the method of choice for confirming a diagnosis. ELISA and microagglutination methods may be more sensitive than tube agglutination testing. A single convalescent titer of 1:160 or greater is considered highly suspect for active disease, but a fourfold rise in titer between acute and convalescent (1–5 weeks) sera is more reliable, since antibodies can persist for many years after infection. Polymerase chain reaction and other molecular biologic techniques have successfully diagnosed tularemia in human specimens. An elevated blood level of creatine phosphokinase may be a clue to tularemia-induced rhabdomyolysis in response to acute infection, especially in highly endemic areas.
Perplexing diseases that are also associated with outdoor and animal exposures, such as psittacosis and Q fever, may be confused with tularemia. Legionnaires’ disease and mycoplasma pneumonia can present with similar clinical courses, without diagnostic sputum. Plague, tuberculosis, and systemic fungal infections produce a spectrum of acute to chronic respiratory manifestations that can mimic pulmonary tularemia.
Treatment and Prevention
Streptomycin was the first effective antibiotic for treating all forms of tularemia, and it remains the agent of choice.29 Gentamicin appears to be equally potent and has the advantage of a broader spectrum of activity if one is initiating treatment when the etiologic diagnosis is less secure.29 In addition, it can be given intravenously, and blood levels can be monitored. Tobramycin, however, appears to be unreliable and therefore should not be substituted for other aminoglycosides. Recent experience confirms that results of therapy are optimal when an aminoglycoside is chosen early in the clinical illness. Tetracycline/doxycycline, ciprofloxacin, and chloramphenicol may be useful alternatives when an aminoglycoside is contraindicated. Relapse rates are higher with the bacteriostatic agents tetracycline/doxycycline and chloramphenicol, especially when given for less than 2 weeks. β-Lactam antibiotics are not effective. The prognosis is excellent with appropriate antimicrobial therapy.
Cautious practices are required when one is dealing with animals and their carcasses. Using gloves, cooking wild animal meat thoroughly, and wearing protective clothing and repellants to avoid bloodsucking arthropods are helpful measures. An attenuated live vaccine strain (LVS) has been available for over 50 years, although it is not fully licensed (in part due to a lack of knowledge regarding the basis of attenuation), and does not offer a high level of protection against respiratory challenge. With the increase in concern over bioterrorism, numerous groups are working to develop an improved vaccine.
 RHODOCOCCUS EQUI
RHODOCOCCUS EQUI
Rhodococcus equi, formerly known as Corynebacterium equi, was first isolated in 1923 by Magnussen when it was identified as a cause of suppurative pneumonia in foals. It was later shown to be a frequent pathogen in horses, cattle, and swine. First described as a pathogen in humans in 1967, the majority of recognized cases in recent years have been in immunocompromised patients, especially individuals receiving corticosteroids, those infected with the human immunodeficiency virus,30 or those who have undergone solid organ transplantation.31
Bacteriology, Ecology, Epidemiology, and Pathogenesis
Rhodococcus is a pleomorphic gram-positive bacillus in the order Actinomycetales. It grows well aerobically on most media, at 37°C, as mucoid pale-pink or salmon-pink colonies that are usually observed by 48 hours of incubation. R. equi has a high cell wall mycolic acid content and is acid-fast, similar to Nocardia spp. and Mycobacteriaceae. Some strains ferment glucose, but the majority will not ferment carbohydrates. Most produce catalase and hydrogen sulfide. β-Lactamase is present in some strains.
The majority of reported cases occurring in humans without HIV infection have been in patients who had significant contact either with livestock (including horses) or soil and environments that were heavily contaminated with livestock waste. In contrast, HIV-related Rhodococcus disease appears to occur in patients who do not have any particular environmental exposure history—implying a wider distribution of the organism.30 There is no known geographic endemicity.
R. equi usually is inhaled, although soft tissue infections after cutaneous inoculation can occur. As an intracellular pathogen it causes disease primarily in patients with impaired cell-mediated immunity and defects in phagocytic processing of organisms. Diseased tissues usually show a necrotizing granulomatous reaction, with histiocytes and macrophages frequently containing bacteria. In contrast to lesions associated with Mycobacterium tuberculosis and systemic fungi, there is also a prominent infiltration of neutrophils in the affected areas, a characteristic shared with other Actinomyces spp.
Clinical and Radiologic Features
Patients frequently complain of indolent symptoms, such as fever, nonproductive cough, and mild dyspnea.31 Typically there is a paucity of findings on physical examination of the chest, but signs of consolidation and pleural friction rubs may be present. Patients with untreated HIV infection generally present in a manner similar to patients without HIV infection, although pleuritic chest pain may be more common. In untreated HIV-infected patients, R. equi disease tends to occur after there has been significant deterioration in the immune system, with CD4 lymphocyte counts less than 200 cells/mm3.30 It is often found associated with other pulmonary infections. Extrapulmonary dissemination occurs in HIV-infected and non–HIV-infected patients, but there appears to be a significantly greater rate of recovery of the organism from blood cultures in HIV patients. The central nervous system is a recognized site of metastatic infection, as it is for Nocardia spp.
The most common radiographic abnormalities are lobar infiltrates, which usually evolve into nodular or cavitating lesions within weeks or months. There is no predilection for involvement of any particular lobe. Pleural effusions are common, occurring in up to 40% of HIV patients with pulmonary R. equi infection. Significant hilar adenopathy is unusual.
Diagnosis and Differential Diagnosis
R. equi can readily be cultured from sputum, bronchial lavage, pleural fluid, or other infected tissue and often from blood. Since the organisms stain as pleomorphic gram-positive bacilli, grow readily on most media, and are usually catalase producers, they can be mistaken for “diphtheroid” or “Coryneform” contaminants unless further testing is done. Therefore it is important for the clinician to alert the microbiology laboratory staff if the possibility of R. equi is entertained. R. equi is slightly acid fast with modified Ziehl–Neelsen stain.
Rhodococci share many microbiologic features with Mycobacteria and Nocardia, and this may account for similarities in the subacute to chronic evolution of the disease. The high mycolic acid content of their cell walls results in their acid-fast staining properties and may also play a role in their similar clinical and pathologic manifestations. Nocardia spp., M. tuberculosis, and nontuberculous mycobacteria also should be considered when acid-fast organisms are found in clinical specimens, especially in immunocompromised patients with nodular or cavitary pneumonia (Table 138-3).
TABLE 138-3 Diagnosing Rhodococcus equi Respiratory Infection
Other considerations in the differential diagnosis of nodular or cavitating pulmonary lesions include malignancy, fungal infection such as Cryptococcus neoformans, anaerobic lung abscess, and necrotizing pneumonia caused by facultative bacteria such as S. aureus or Klebsiella pneumoniae.
Treatment and Prevention
Antibiotic therapy alone is usually adequate to achieve cure.31 As with mycobacterial infections, multidrug regimens and therapy of at least 2 to 6 months’ duration may be needed, especially in immunocompromised patients. Because R. equi
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree