Chapter 83
Wound Care
William A. Marston
As the populations of developed countries age and become more sedentary, the incidence of diabetes and obesity increases, along with a dramatic increase in the incidence of chronic nonhealing wounds.1 In most medical communities, surgeons are considered the local experts in the care of wounds and are expected to manage cases that do not respond to typical treatments recommended by primary care clinicians. This chapter focuses on the diagnosis and management of chronic wounds of the extremities that are typically referred to vascular surgeons for definitive management.
The most common etiologic factors in nonhealing limb ulcers are chronic venous insufficiency, arterial occlusive disease, and diabetic neuropathy. Most reports suggest that the most common cause is venous insufficiency, but patients with arterial insufficiency and diabetic neuropathy are at the highest risk of limb loss.2 Accurate identification and treatment of these underlying disorders is the most important determinant of ulcer healing.
Recently, specialized wound clinics have been created, promising major benefits in wound healing, limb salvage, and quality of life. In some cases, these centers effectively coordinate the efforts of diverse specialists to improve the treatment of difficult patients, but in other cases, the centers have been dominated by an interest in profitability. It is clear that a vascular specialist is critical to the optimal management of wounds; no other specialist can provide the comprehensive management of arterial and venous insufficiency required by the majority of patients treated in wound centers. Similarly, a vascular surgeon participating in a multispecialty wound center can expect assistance from other valuable specialists, including endocrinologists, plastic or orthopedic surgeons, podiatrists, orthotists, and physical therapists, to maximize positive outcomes for their patients.
Normal Wound Healing
Healing of acute wounds normally proceeds through well-defined phases of hemostasis, inflammation, proliferation, and remodeling. Although these phases are typically described separately, the process is actually a gradual progression guided and regulated by the complex interaction of platelets, neutrophils, macrophages, and other cells that respond to and produce growth factors, cytokines, proteases, and inhibitors.3 Although the effects of many of these proteins have been described, the deficiencies that lead to the failure of timely healing are not well described. It is clear that all of the common causes of nonhealing wounds, including venous hypertension, arterial insufficiency, chronic pressure, and chronic inflammation, inhibit this orderly healing process, usually in the inflammatory stage, with little meaningful tissue proliferation demonstrated.
Inflammatory Phase
The inflammatory phase of wound healing is critical to the normal healing process. Mediated by mast cells, neutrophils, and macrophages, inflammation develops within 24 hours of acute wounding and continues for up to 2 weeks. The involved cells produce chemokines, cytokines, and growth factors that mediate the inflammatory process.4 Cytokines such as tumor necrosis factor-α (TNF-α), interferon-δ (IFN-δ), and the interleukins (ILs) are released by activated lymphocytes and macrophages into the tissue, resulting in further recruitment and activation of fibroblasts and epithelial cells in the wound.5 Neutrophils following chemoattractant signals migrate into the wound, releasing concentrated matrix metalloproteinases (MMPs). The MMPs are responsible for clearing damaged tissue from the wound area by breaking down collagen (MMP- 1 and -8), gelatin (MMP-2 and -9), and elastin (elastase).6 The activity of MMPs is closely controlled by tissue inhibitors of MMPs (TIMPs), which are produced principally by macrophages.
Proliferative Phase
In the proliferative phase, growth factors, including platelet-derived growth factor (PDGF), transforming growth factor-β (TGF-β), and vascular endothelial growth factor (VEGF), are secreted by multiple cell types but primarily by macrophages. They are responsible for the recruitment of fibroblasts to commence the proliferative phase and for the initiation of angiogenesis required to support tissue generation and epithelial growth.7 Fibroblasts responding to signaling growth factors and cytokines migrate into the extracellular matrix, synthesizing collagen and proteoglycans and developing granulation tissue to fill the wound. Orderly capillary growth and networking must occur to support the developing granulation tissue; this requires a complex interplay of growth factors, including multiple isoforms of VEGF, TGF-β, and hypoxia-inducible factor-1 (HIF-1).8 It is believed that HIF-1 is a key mediator of the effects of other angiogenic growth factors. In response to reduced levels of oxygen, HIF-1 stimulates the production of all isomers of VEGF and other growth factors active in the stimulation process.9 MMPs are also required for the normal progression of angiogenesis and must be secreted at the correct time by budding capillary loops to allow them to degrade the existing capillary basement membrane as they sprout to establish a new capillary channel.10
Epithelialization and Remodeling
Epithelialization occurs most rapidly on a well-granulated, confluent tissue bed, in response to signals from growth factors, such as epithelial growth factor and keratinocyte growth factor. Epithelial cells migrate into the wound from the periphery by secreting MMPs to degrade the nonviable tissue at the wound edge, allowing migration into the wound. Normally, epithelial migration should continue until other epithelial cells are contacted.11
Remodeling is a long-term process in which type III collagen is largely replaced by mature type I collagen. This process also requires the presence of MMPs to degrade the type III collagen in a controlled fashion mediated by TIMPs, facilitating its replacement with maturing type I collagen.12
Mechanisms of Abnormal Wound Healing in Chronic Wounds
Chronic nonhealing wounds occur when the normal healing process is disrupted. This is most frequently the result of an underlying disorder that causes a prolonged, unchecked proinflammatory state. These disorders include venous hypertension, chronic pressure, bacterial colonization, inadequate tissue perfusion, and cellular senescence.
Inflammation
Recurrent or prolonged inflammatory stimuli result in chronic wounds that are characterized by persistent upregulation of proinflammatory cytokines and MMPs. Although this environment is necessary for a brief period for acute wound healing, persistence of this environment has detrimental effects. Wound fluid collected from chronic nonhealing ulcers has been reported to inhibit DNA synthesis and mitotic activity of normal skin fibroblasts and keratinocytes.13 In contrast, fluid collected from acute wounds stimulate these measures of cellular activity. Chronic wound fluid may contain high levels of MMP-2 and MMP-9 and abnormally low levels of TIMPs.14 In venous ulcers, MMP levels decrease after compression treatment. In a study of 56 patients with pressure ulcers, Ladwig et al15 found that the ratio of MMP-9 to TIMP-1 was associated with wound healing outcomes: an elevated concentration of MMP-9 in relation to TIMP-1 was predictive of poor healing. Although this upregulation of protease levels might be expected to degrade endogenous growth factors, Drinkwater et al16 reported that the level of VEGFs in venous ulcer tissue was not decreased and was persistently high compared with normal tissue, and was equivalent to levels in tissue from acute wounds. In a separate study, these researchers found that fluid from venous ulcers inhibited angiogenesis, postulating that this effect must be caused by the presence of inhibitors or a lack of available receptors rather than an absence of stimulatory growth factors.17 Patients with critical limb ischemia (CLI) also demonstrate deficits of growth factor function. Palmer-Kazen et al18 found that distal limb tissue in patients with CLI had lower levels of VEGF compared with nonischemic proximal tissue in the same limb.
Cytokines
Upregulation of proinflammatory cytokines, including TNF-α, IL-1, and IL-6, has also been described in chronic venous wound fluid.13 These levels were found to improve when ulcers began to heal, with a decrease in size and improvement in granulation. Diabetic foot ulcers may also display proinflammatory upregulation and low expression of angiogenic factors.19
Cell Senescence
Cellular senescence has been reported in fibroblasts collected from chronic nonhealing ulcers. Lal et al20 found that fibroblasts from patients with increasing levels of venous disease by clinical class, etiologic, anatomic, and pathophysiologic (CEAP) criteria displayed a progressively diminishing response to agonist-induced proliferation. Fibroblasts from CEAP class 6 patients displayed severe inhibition of the proliferative response to TGF-β1. Mendez et al21 reported a series of studies of cultured fibroblasts from venous ulcers indicating that these typically display phenotypic characteristics of cellular senescence, including slow growth and altered morphology. Stimulation of senescent fibroblasts may be a key target of therapy for improving the healing potential of chronic leg ulcers.
Therapeutic Targets
The development of therapeutic targets based on the known mechanisms of poor healing has been difficult, given its overall complexity. Initial strategies at modulating protease expression have not yielded major improvements in healing. A dressing composed of a combination of collagen and regenerated cellulose (Promogran, Systagenix, North Yorkshire, United Kingdom) has been developed based on its ability to bind MMPs in wound fluid in vitro. In a study of 33 patients with diabetic foot ulcers, ulcers treated with Promogran exhibited a decreased ratio of MMP-9/TIMP-2 in tissue biopsy compared with ulcers not treated with the material.22 However, in a prospective randomized study of 276 patients with diabetic foot ulcers, treatment with Promogran for 12 weeks resulted in complete wound closure in 37%, compared with 28% treated with saline-moistened gauze, a difference that was not statistically significant.23
General treatments for the reduction of inflammation such as doxycycline and nonsteroidal anti-inflammatory drugs have been studied in animal models and considered for clinical use. Doxycycline at low doses was found to be useful in stimulating improved periodontal wound repair in several studies of patients with chronic periodontitis.24 Also, in a pilot study of chronic venous leg ulcers, high-dose doxycycline resulted in improvement of recalcitrant wounds and reduced wound fluid MMP levels.25 Larger studies of the effect of doxycycline on wound healing are currently underway. Some researchers believe that the simple inhibition of upregulated proteases or cytokines is unlikely to yield significant gains in healing. As evidenced by the importance of MMPs in multiple aspects of the normal healing process, gross inhibition of their function impairs capillary development, epithelial migration, and collagen maturation. Beidler et al,26 in an analysis of cytokine levels and venous ulcer healing, found that untreated ulcers with higher levels of proinflammatory cytokines, including IL-1, IL-12p40, and IFN-δ, healed significantly better than those with lower levels of these cytokines before compression. It is clear that strategies to address the abnormal progression of healing in chronic ulcers must consider that inflammation is necessary at various points in the process and must be suppressed at other stages. Currently, no diagnostic modalities are available to rapidly test the MMP or cytokine levels in wound fluid or tissue in a nonresearch setting, but clinically relevant testing strategies are under development.
Etiology of Ulceration
The successful treatment of patients with nonhealing leg ulcers requires that the underlying cause of ulceration be correctly diagnosed. Common differential diagnoses of leg ulceration are provided in Table 83-1. It has been reported that multiple causes are involved in more than 20% of leg ulcers, so it is clear that each potential diagnosis must be considered in every patient.27 Based on the history and physical examination, the proper cause or causes of chronic ulcers can be defined in the majority of patients, but if doubt remains, confirmatory noninvasive testing should be performed.
Table 83-1
Differential Diagnosis of Causes of Chronic Nonhealing Leg Ulcers
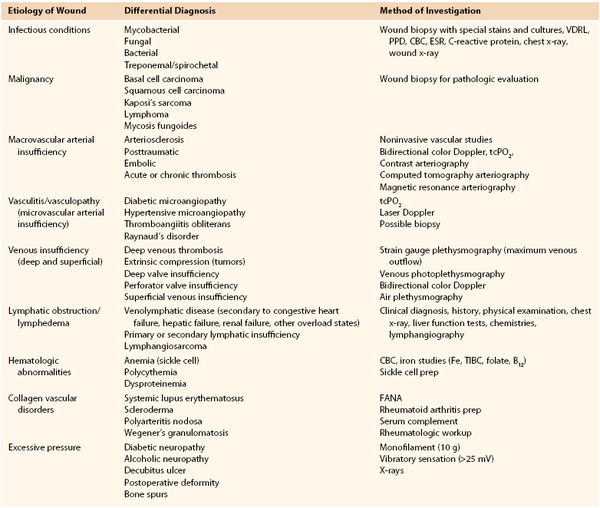
CBC, Complete blood count; ESR, erythrocyte sedimentation rate; FANA, fluorescent antinuclear antibody test; PPD, purified protein derivative; tcPO2, transcutaneous oxygen tension; TIBC, total iron-binding capacity; VDRL, Venereal Disease Research Laboratory.
Venous Leg Ulcers
The epidemiology and pathophysiology of venous leg ulcers are described in Chapter 55. Risk factors related to the development of venous ulcers are the same as those for the development of venous disease in general. It is important to understand that venous disease in any combination of anatomic sites may result in limb ulceration, including superficial venous insufficiency alone. The anatomic sites of venous reflux defined by duplex ultrasound examination in a series of 138 patients with venous leg ulcers are listed in Table 83-2.28
Table 83-2
Anatomic Distribution of Venous Reflux in 138 Patients28
| Venous System | Limbs (%) |
| Deep alone | 43.5 |
| Deep and superficial | 21.0 |
| Deep, perforator, and superficial | 6.5 |
| Superficial alone | 18.1 |
| Superficial and perforator | 10.9 |
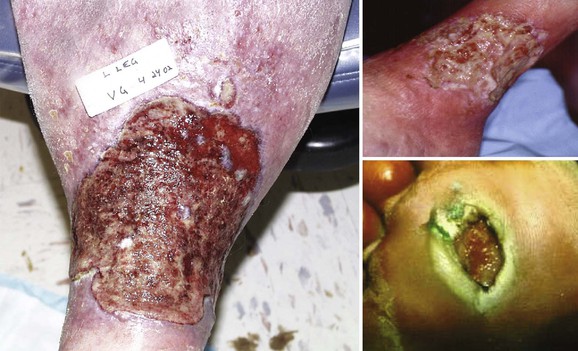
The pathway between chronic venous hypertension and limb ulceration has been debated for years. Currently, many investigators believe that venous insufficiency results in a chronic, recurring condition resulting in years of inflammatory upregulation in the soft tissues of the lower extremity. The effects are most severe at the ankle, but the foot is spared because of the construction of the fascial compartments and the lack of major veins in the deep tissues. Proinflammatory cytokines are chronically upregulated, resulting in the overexpression of proteases, leading to increased tissue fibrosis and the clinical appearance of lipodermatosclerosis (Fig. 83-1). Ulceration eventually occurs either spontaneously or due to minor limb trauma in the lower calf or ankle that cannot heal.
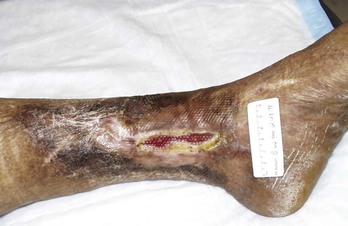
Figure 83-1 Limb with chronic venous insufficiency illustrating wound tissue scarring and lipodermatosclerosis.
Based on the known information about the cause of venous leg ulcers, strategies for management have traditionally involved techniques designed to either eliminate venous hypertension through the correction of venous insufficiency or counter venous hypertension through compression of the limb. Specific strategies for inhibiting the pathway leading from hypertension to ulceration have been proposed and are under study, but are not available clinically at this time.
Diabetic Foot Ulcers
The epidemiology and pathophysiology of diabetic foot ulcers are reviewed in detail in Chapter 116. For management purposes, it is critical to remember that the underlying causes of ulcers, such as polyneuropathy, vascular insufficiency, and infection, must be addressed before specific wound treatments can assist in healing. Inadequate pressure offloading is the most common cause of failure to heal, and no active wound therapy can overcome persistent pressure on a plantar ulcer. The assistance of an expert orthotist and consistent patient education to achieve compliance are far more important to the healing of diabetic foot ulcers than the specific dressing or agent selected.
Limb Ulcers Associated with Arterial Insufficiency
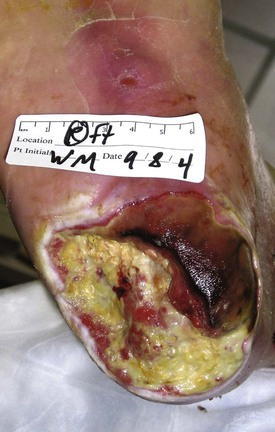
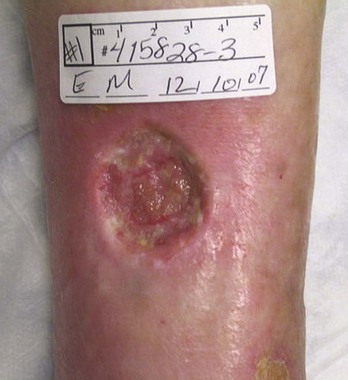
The treatment of patients with chronic nonhealing leg ulcers associated with arterial insufficiency involves a critical assessment of the patient’s suitability for invasive procedures and the potential risk of limb loss based on ulcer characteristics. Data from several clinical trials studying critical limb ischemia have identified a risk of major amputation in 25% to 40% of patients at 1 year if revascularization is not performed.29,30 These studies typically enroll patients with pain at rest and tissue loss, and tissue loss patients appear to have a higher incidence of limb loss over time. The clinician is faced with a variety of wound presentations, from a superficial partial-thickness wound (Fig. 83-2) to a deep, complicated leg ulcer involving tendon or bone (Fig. 83-3), to varying amounts of pedal gangrene. Many clinicians believe that these patients require different treatment plans, but there is limited information stratifying outcomes based on wound characteristics.
Figure 83-2 Wagner grade 1 diabetic foot ulcer. The wound penetrates through the full thickness of the skin, involving the subcutaneous tissue but not the deeper tissue layers.
In a recent study of 169 limbs with chronic ulcers and arterial insufficiency, a protocol was reviewed in which patients were treated initially with conservative wound care, including débridement, pressure offloading, and moist wound healing in a multispecialty wound center.31 Patients were not treated initially with revascularization because of concomitant medical problems (44%), poor functional status (34%), lack of a target vessel in the distal limb (14%), or patient refusal (8%). At 1-year follow-up, 52% of limb ulcers were healed, and 23% required amputation. Life-table analyses of limb amputation and ulcer closure are presented in Figure 83-4. Risk factor analyses revealed that the ankle-brachial index (ABI) and wound grade were associated with amputation at 1 year. Thirty-four percent of limbs with an ABI of less than 0.5 and 43% of limbs with an ABI less than 0.4 required amputation at 12 months, compared with 15% of limbs with an ABI between 0.5 and 0.7. From this review, the authors concluded that patients with limited wounds and at high risk for a poor outcome with revascularization can be reasonably treated without revascularization, and many will experience limb salvage. Revascularization should be considered in those who do not improve after a trial of noninvasive treatment. In those with more severe wound grades, including patients with pedal gangrene, revascularization should be considered early to improve the chance of limb salvage.
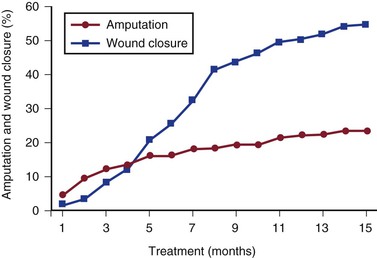
Figure 83-4 Incidence of major amputation and complete wound closure by life-table analysis in 169 limbs with tissue loss and nonrevascularized arterial insufficiency.31
Arterial insufficiency is a common complicating factor for limb ulcers associated primarily with other causes, including venous insufficiency, diabetes, and rheumatoid arthritis. It is important that the status of the arterial supply be determined in every patient at the initial evaluation.
Other Causes of Limb Ulceration
Although the preceding causes are important factors in more than 90% of nontraumatic limb ulcers, other causes, including vasculitis, sickle cell anemia, malignancy, and dermatologic disease, are routinely seen by vascular specialists. It is beyond the scope of this chapter to review these leg ulcers in detail. The key to diagnosing these atypical causes is ulcer biopsy. Many of these conditions may be confused with venous ulceration and may coexist in a patient with chronic venous insufficiency. The most important diagnosis to make is Marjolin’s ulcer, which may develop when squamous transformation occurs in a preexisting benign chronic wound (Fig. 83-5). This neoplasm is often not detectable by observation, so the physician must maintain a high index of suspicion. Whenever a wound does not respond to a comprehensive treatment plan as expected or a wound that has been healing ceases to progress for no known reason, a biopsy should be performed to rule out malignancy. Further information on the diagnosis and management of atypical wounds is beyond the scope of this chapter.
Clinical Presentation and Initial Evaluation
The primary cause of most wounds can be identified after the history and an attentive physical examination. Figure 83-6 contrasts common types of lower extremity ulcerations.
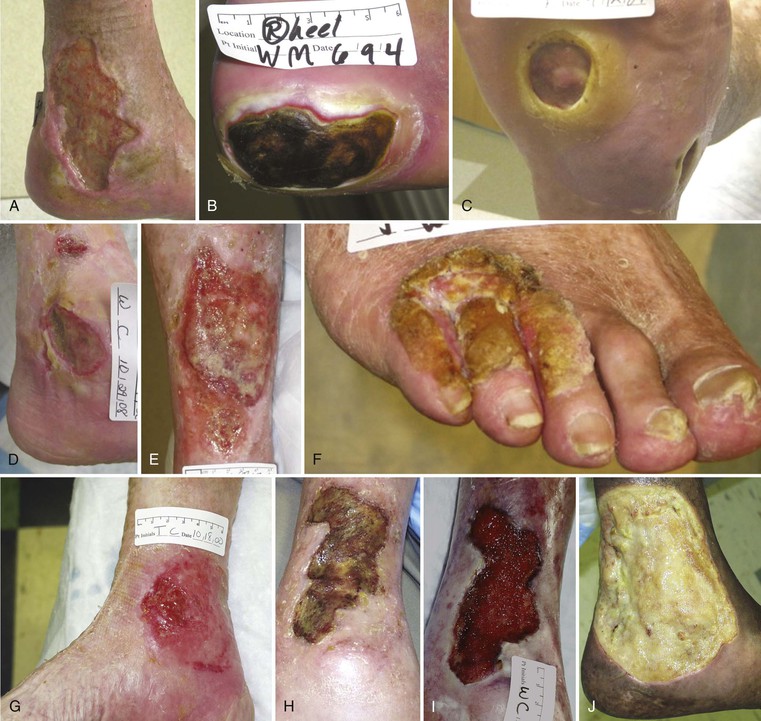
Figure 83-6 A, Leg ulcer associated with chronic venous insufficiency. B, Diabetic neuropathic ulcer. C, Plantar ulcer in a patient with diabetes and chronic Charcot’s neuropathy. D, Leg ulcer in a patient with both arterial and venous insufficiency. E, Leg ulcer in a patient with systemic lupus and venous insufficiency. F, Squamous cell carcinoma arising in a chronic foot ulcer. G, Leg ulcer associated with rheumatoid arthritis. H, Bacterial biofilm covering the surface of a nonhealing venous leg ulcer. I, Same ulcer depicted in H after elimination of the biofilm following débridement and 2 weeks of antibiotic therapy. J, Chronic leg ulcer in a patient with sickle cell anemia.
Wound Classification Systems
The most widely recognized system for wound classification is the Wagner grading system. Originally intended to classify diabetic foot ulcers, it is useful to describe any complex lower extremity wound. Limbs with higher Wagner grades, particularly grades 3 to 5, are at increased risk for limb loss (Table 83-3).31
Table 83-3
Incidence of Limb Amputation in Patients Presenting with Limb Ischemia Based on Wagner Grade* at Initial Presentation31
| Grade | Criterion | Amputation at 12 Months (%) |
| 0 | Preulcerative lesion | |
| 1 | Superficial ulcer | 12 |
| 2 | Ulcer extending deep to tendon, bone, or joint | 19 |
| 3 | Deep ulcer with abscess or osteomyelitis | 31 |
| 4 | Forefoot gangrene | 49 |
| 5 | Whole foot gangrene | 100 |
* Grades 1-4 (n = 169).
Wound Size Measurement
At each visit it is important to measure the wound size to document progress and alter the treatment plan if necessary. The busy physician evaluating multiple patients with limb ulcers should use such measurements early after intervention or treatment initiation to determine whether the wound is responding to treatment or is worsening. Subtle changes in the wound itself, including the development of inflammation or bacterial colonization, may manifest only as the suppression of healing. Routine wound measurement allows the consideration of alternative treatment methods as soon as progress in wound healing ceases.
Numerous methods are available to document and follow wound size, including digital photography with computerized planimetry, direct planimetry, and simple measurement of dimensions. Measurement of wound dimensions, including depth, should be performed at each clinic visit. Software systems are also available to allow the determination of wound size from calibrated digital photography.
Wound Bed Assessment and Preparation
Once the history and physical examination are completed and a general idea of the underlying cause of the wound is determined, the wound itself must be assessed for local factors inhibiting healing. Numerous factors must be identified and corrected to optimize healing potential, a process termed wound bed preparation.32 The components of this process include débridement of nonviable tissue, identification and correction of bacterial involvement, control of chronic inflammation, elimination of limb edema, and control of wound exudate. The first two components are addressed here.
Débridement
The majority of chronic limb ulcers are covered with nonviable tissue, including callus, eschar, fibrinous material, and slough. This tissue has no regenerative capability, harbors bacteria, and prevents the migration of healthy epithelium into the wound. Débridement is required to excise nonviable tissue and should be aggressive, particularly at the initial evaluation. Cellular senescence has been identified in wound tissue around diabetic foot ulcers and chronic venous ulcers of long duration, indicating the need for wound excision to remove all surrounding tissue in these cases (Fig. 83-7).33 In a prospective multicenter study of becaplermin for the healing of diabetic foot ulcers, a secondary analysis suggested that centers employing more frequent wound débridement achieved better healing rates.34
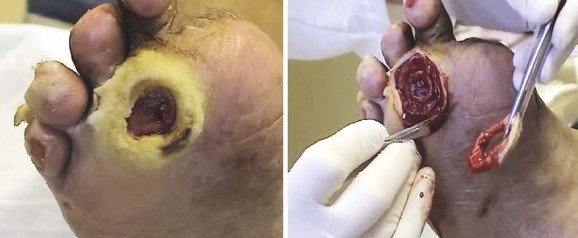
Figure 83-7 Example of aggressive débridement of senescent tissue in and around the wound bed to create an acute wound out of a chronic wound.
In a retrospective review of 676 patients enrolled in clinical trials of novel therapeutic agents for venous or diabetic ulcers, the incidence of wound débridement was studied and correlated with wound closure.35 Thirty-three centers where limbs were débrided more frequently were associated with higher rates of wound closure for both types of wounds, but an increased frequency of wound débridment per patient did not statistically correlate with higher rates of wound closure. Based on this information, it appears that débridement for chronic wounds is important to eliminate nonviable tissue, and may be required multiple times during the treatment course of the wound. However, the current body of evidence provides little guidance on the question of how often diabetic foot wounds should be débrided or the best timing, such as weekly, monthly, or other.
Numerous alternatives to surgical débridement have evolved, including chemical débridement, ultrasound débridement, hydrodébridement, and larval therapy. In general, there are two situations in which alternatives to surgical débridement may be desirable: patients in long-term nursing facilities, and those who have difficulty traveling to wound or surgical clinics for frequent surgical débridement. These patients may benefit from chemical débridement or some other method.
Chemical.
Chemical débriding agents are typically composed of enzymatic agents, including collagenase (Santyl, Healthpoint Ltd, Fort Worth, Tex) or papain-urea (Accuzyme, Panafil, Healthpoint Ltd). The papain-urea agents were recently removed from the U.S. market, leaving collagenase as the primary chemical agent available for use. Collagenase may be used as a maintenance débriding agent to remove moderate amounts of fibrinous slough from the wound surface, but it is usually ineffective against thick tissue or eschar.
Larval.
Larval therapy using medical maggots has been studied as an alternative to conventional therapy in several nonrandomized studies. Sherman reported that 80% of maggot-treated pressure wounds achieved complete débridement, compared with 48% of conventionally treated wounds.36 However, most of the studies evaluating maggot therapy have used autolytic or chemical débridement as comparators. A randomized study of larval therapy compared with hydrogel for the management of chronic leg ulcers with at least 25% of the wound surface affected with slough or necrotic material was completed and reported in 2009.37 All other wound therapies were similar for each group. The study found that larval-treated wounds experienced significantly faster débridment than the hydrogel-treated group, but at the expense of significantly higher ulcer-related pain scores. No difference in ulcer healing or patient quality of life was demonstrated.
Ultrasound
Débridement using standard surgical techniques may be particularly painful, leading to interest in alternative methods that achieve wound débridement while increasing patient comfort. Several energy modalities, including ultrasound, have been investigated as adjuncts to débridement. Ultrasound may be used in conjunction with a surgical instrument: the instrument contacts the wound to deliver ultrasound energy to the tissue to loosen and remove nonviable tissue. Alternatively, the use of saline mist avoids the need for direct contact with the wound surface. In a study of the noncontact method of ultrasound treatment, mist ultrasound was compared with standard treatment of diabetic foot ulcers. Although there was no significant difference in the rate of ulcer healing based upon the intent-to-treat analysis, there was a significant increase in the rate of healing in patients who completed a course of properly applied mist ultrasound treatment.38 Ultrasound débridement may be associated with less pain than surgical methods; however, no studies have compared the efficacy of ultrasound débridement with that of surgical methods.
In summary, no other form of débridement has demonstrated superiority to standard surgical débridement, and most of these methods are less cost effective in the majority of cases. Alternative methods of débridement are generally recommended when surgical débridement is not possible or is not desirable.
Bacterial Colonization
Significant bacterial infection is a common complication in chronic wounds. In several prospective randomized clinical trials of therapies for diabetic foot ulcers, wound infection requiring systemic antibiotic therapy occurred in 20% to 25% of cases.39,40 However, the treatment of bacteria in wounds that do not appear to be clinically infected is controversial. Moreover, it is believed that uninfected healing wounds are colonized with bacterial flora. Treatment with antibiotics is not indicated, so routine culturing of wounds in these cases is not recommended. The eradication of normal skin and nonvirulent wound bacteria is not desirable and may support the growth of more virulent and resistant bacterial strains. Of note, however, recent studies have identified an increase in the incidence of resistant organisms from cultures of chronic nonhealing ulcers that do not respond to standard treatment protocols.41
Although not fully characterized, the concept of biofilm involvement has developed to describe the bacterial colonization of wounds. Biofilms harboring bacteria are well known to cover indwelling catheters, industrial surfaces, and dental structures. Bacteria in these situations secrete a polysaccharide substance that covers the colonies, which grow and interact to inhibit host-generated control mechanisms. Mechanical methods of cleansing these surfaces can minimize biofilms, but they typically repopulate rapidly after cleansing. In chronic wounds, biofilms appear to multiply to the point at which they inhibit healing by stimulating chronic inflammation, inactivating growth factors critical to the healing process, and preventing orderly angiogenesis required for healing (Fig. 83-8).42 If untreated, it is believed that biofilms will continue to inhibit wound healing or may lead to more significant systemic infection.
Given the emergence of this clinical problem, wounds with poor progress and those demonstrating any evidence of enlargement or infection should be evaluated with quantitative cultures. If cultures demonstrate greater than 105 bacterial counts per cubic millimeter, the patient should receive systemic therapy guided by bacteria-specific sensitivities.43
In general, the management of bacterial colonization of chronic ulcers remains a controversial area with limited high quality research and contradictory results in the studies available for review. Although there is general agreement among experts that wounds with obvious signs of clinical infection should be treated with systemic antibiotics, there is no consensus on the management of wounds colonized with bacteria or bacterial biofilms without signs of systemic infection. The definition of a critically colonized wound is not universally standardized and may vary depending on the virulence of the colonizing bacteria. Recent studies have described the types of bacterial biofilms that may develop in chronic wounds, with multiple species organized into colonies on the wound surface that are resistant to topical therapies. This adds complexity to treatment, and information on the clinical relevance of these biofilms and the best strategies to treat them is not yet well developed. Finally, the changing nature of bacterial behavior over time may render studies performed as recently as 5 to 10 years ago irrelevant at the present time. The importance of the bacteria affecting the wound bed remains controversial. Although some studies have reported that the information provided by wound cultures is of little prognostic significance,44 others report improved healing with specific antibiotic treatment of cultured bacteria.45 Diagnostic methods are becoming clinically available using polymerase chain reaction to provide more detailed descriptions of the bacteria existing in the wound bed.46 This information may allow more specific tailored antimicrobial strategies to treat each individual wound. However, use of these methods has not yet been established to improve outcomes in the care of chronic wounds.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree