Chapter 106
Brachiocephalic Artery
Endovascular Treatment Diseases
Daniel G. Clair, Jessica M. Titus
Occlusive disease of the arch vessels can have a dramatic impact on a patient’s neurologic function and ability to perform normal daily functions using their upper extremities. It also can have a significant effect on their medical care because accurate blood pressures often cannot be monitored, thus making it difficult to routinely and reliably measure the affects of therapeutic strategies for treating hypertension or hypotension in both acute and chronic situations.
Initial interventions for brachiocephalic occlusive disease were solely surgical, involving revascularization with prosthetic graft or endarterectomy of the involved vessel.1,2 Although early reports of open surgery demonstrated excellent results, the technical skills required and the potential need to deal with problems related to intraoperative difficulties stimulated searches for a less invasive method to treat these patients. Initial reports of endovascular interventions in the arch vessels consisted of small numbers of patients treated with angioplasty and limited follow-up.3–6 However, it was clear from these studies that the option of endovascular intervention was reasonable and efficacious. Further advancements in the endovascular armamentarium, including both the introduction of stents for the arch vessels and newer technical strategies, have made endovascular therapy the primary approach for many patients with arch vessel occlusive disease. In skilled hands, the risks are low and the morbidity is minimal. However, it is one of the more complex endovascular interventions to perform, and the consequences of poor technique can be devastating.
Procedure Planning
A great deal of the information on patients with occlusive disease of the arch vessels comes from surgical series,7–10 and although much of the demographic information can be gleaned from these series, they also offer insight into the risks related to surgical reconstruction, including both direct anatomic and extraanatomic approaches.
A detailed understanding of the anatomy of the aorta arch along with clear knowledge regarding the positions of the vessel origins is a necessity for planning these procedures. A thorough understanding of the nature of the disease being addressed is also important. The most common cause of arch vessel stenosis is atherosclerosis, but even this occurs relatively infrequently. In evaluations for carotid occlusive disease, only approximately 5% to 15% of patients are found to have disease in the arch vessels.11–14 The incidence in patients without cerebrovascular disease is lower, but it is not insignificant in the general population, with an incidence of approximately 1.5% to 2.0%. In patients with coronary disease,15,16 it can be as high as 4% to 7%,17,18 with increasing incidence (up to 11%) in those with more significant coronary disease. The left subclavian artery is involved most often, followed by the innominate and left common carotid arteries, which are both affected approximately 30% of the time.12,19 Other less common causes of arch vessel disease include Takayasu’s arteritis, radiation-induced injury, aneurysm, and dissection.
Imaging Studies
Computed Tomographic and Magnetic Resonance Angiography
The most commonly used methods for assessing the anatomy of the aortic arch are computed tomographic angiography (CTA) and magnetic resonance angiography (MRA), along with standard angiography (see Chapter 98). The axial imaging modalities have the obvious advantage of avoiding catheter manipulation within the arch and can detect both aneurysmal disease and mural atherosclerotic debris; the latter can have important implications (e.g., in thoracic endovascular aortic repair planning) and for assessing risk of a catheter-based approach. These studies can also assess the extent of calcification at the origins of the vessels, and when the imaging region includes the brain, can define the intracranial circulation as well. In most instances, the source images can be reformatted into three-dimensional models of the aortic arch and its branches to allow excellent visualization of the anatomy both within and above the arch. The information obtained from these studies can often allow complete procedural planning before performing conventional angiography.
The normal anatomic make-up of the aortic arch involves three arch vessels: the innominate or brachiocephalic artery, which normally gives rise to the right subclavian and right common carotid arteries; the left common carotid artery; and the left subclavian artery. However, in up to 20% of individuals, the left common carotid artery may originate from the innominate artery, thus leaving only two main trunks off the aortic arch, commonly referred to as a bovine arch (see Fig. 105-2).20 In as many as 6% of individuals, the left vertebral artery may originate from the aortic arch directly, between the left common carotid and left subclavian arteries. Finally, aberrant origin of the subclavian arteries, in particular, with or without associated Kommerell’s diverticulum (see Chapter 140) and/or a right sided aortic arch, are best displayed on preprocedural axial imaging studies.
Arteriography
Arch angiography is usually the next step in imaging to assess the arch vessels and the extent of disease. Care must be taken when performing this procedure because the occlusive process in the vessels usually extends into the aortic arch, and debris can be dislodged by simply placing a wire across the arch. Flush aortography is generally performed with a pigtail or halo catheter, rather than a straight flush catheter, because a straight catheter can cause dissection when high-pressure injections are performed with the catheter through the arch. The imaging is best performed in the left anterior oblique projection so that the arch can be “opened up” on the imaging screen. Frequently, it is helpful to rotate the imaging acquisition arm with a wire in place across the arch so that the optimal projection to fully open the arch can be identified. The images obtained from this aortogram are used to help assess the location of the vessel origins, the degree of vessel stenosis, and the type of catheter or guide that will be necessary to access and image the vessel origin.
Angiographic imaging of the origins of the right common carotid and right subclavian arteries is best obtained with selective catheterization of the innominate artery origin and imaging in the right anterior oblique projection at approximately 20 degrees. This particular orientation will open up the origins of the innominate branches to afford ideal visualization for treating either origin. Comparison of CT-rendered, three-dimensional images and angiography provides a better understanding of the information that can be gained before angiography is completed (Fig. 106-1).
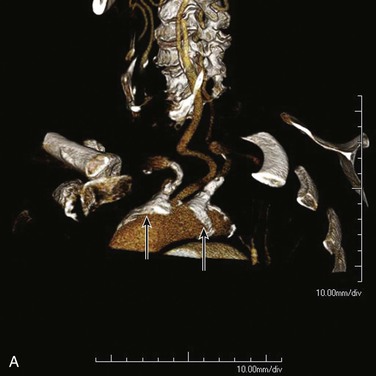
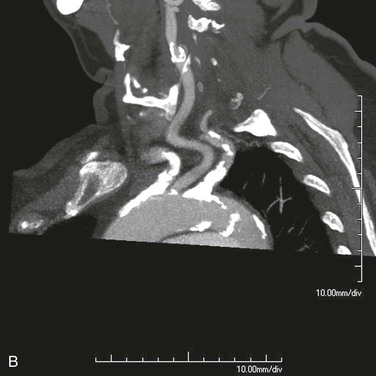
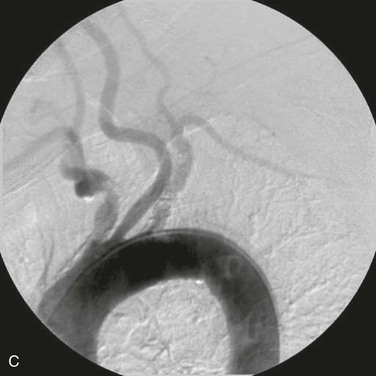
Figure 106-1 A, CT three-dimensional surface rendering of the aortic arch showing severe calcific disease of the origins of the innominate and left subclavian arteries (arrows). B, Maximum intensity projection images of the same patient reconstructed from the CT scan. C, Arteriogram of the same patient. Note the extensive calcification at the origins of the innominate and proximal left subclavian arteries.
Transesophageal Echocardiography
Transesophageal echocardiography is another method that has been used to image the arch, but it has not been used frequently in planning arch interventions.21 This imaging modality, in particular, can give real-time information regarding the presence and extent of dissection in this region and the mobility of atheromatous plaque near the arch vessel origins. Although it is not commonly thought of as an imaging method that is necessary for these procedures, transesophageal echocardiography can provide important information, especially in the setting of complex arch disease.
Anatomic Considerations
Anatomic features that make performance of arch vessel intervention easier or more difficult are briefly highlighted in Box 106-1. With an understanding of normal anatomy, common variants, and the information obtained from preinterventional diagnostic studies with respect to the severity and extent of disease, a plan can be formulated for treatment. Among the variety of preoperative imaging modalities available, preference should be given to the best available method at the practitioner’s facility. CTA offers better anatomic information and can allow assessment of the extent of calcification in the arch vessel origins.
Normal arch anatomy places the origins of the arch vessels at the most superior aspect of the arch. In this configuration, the easiest of the arch branches to access is the left subclavian artery, and the most difficult to access is often the innominate artery. In the setting of a common origin of the left common carotid artery and the innominate artery, access to the left common carotid artery can be more challenging. Additionally, rotation of the vessel origins around the arch toward the ascending aorta can make access and intervention much more challenging, and in some cases, such rotation may make surgical treatment of the disease preferable (Fig. 106-2).
Figure 106-2 More proximal origin of aortic arch branches, as shown here for a type III arch, makes catheter access to the arch vessels difficult.
Disease Distribution
Knowledge of the extent of disease within the vessels is important when planning arch interventions. Complete occlusion of these vessels have proven difficult to re-canalize in a significant number of patients, with published series reporting failure rates ranging from 0% to 40% when trying to re-canalize occluded subclavian arteries.22–25 It is important to be aware of how the arch itself is affected by occlusive disease and the relationship of critical branches to the lesion being treated. For example, when intervening on the left subclavian artery, to avoid injury to the vessel, it is imperative to know the location of the origin of the left vertebral artery and its proximity to disease, whether it is directly off the arch near the subclavian origin or from the normal anatomic position off the proximal subclavian artery. Additionally, it is essential to recognize that intervention in the origin of any vessel can have an impact on the orifice of closely neighboring vessels (Fig. 106-3). This is particularly important when treating the origin of an innominate artery near the left common carotid artery, the origin of either the right common carotid artery or the right subclavian artery because treatment of one can affect the patency of the other. All these situations are best addressed by understanding the anatomy ahead of time and planning appropriately.
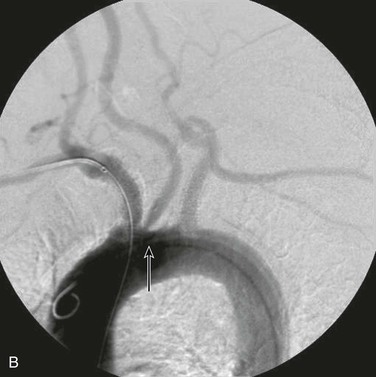
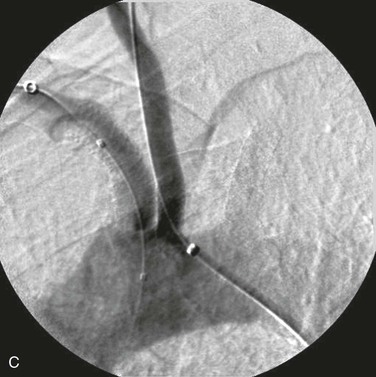
Figure 106-3 A, Treatment of a neighboring arch vessel can force treatment of an adjacent arch branch. After treatment of the innominate artery, the left common carotid can be impinged on by a shift of aortic plaque, thus necessitating intervention in the left common carotid origin. B, A successfully treated innominate artery has shifted aortic plaque, which resulted in stenosis at the origin of the left common carotid artery (arrow). C, Treatment of the common carotid origin and simultaneous protection of the innominate artery’s stented origin.
Options for Endovascular Therapy
Angioplasty
Initial descriptions of endovascular interventions in the supra-aortic trunks involved angioplasty alone.3–6,26–30 In the majority of these patients, initial success rates were in the range of 80% to 95%, and recurrence rates were acceptable and varied from 8% to 25% at 1 to 2 years in those in whom immediate technical success was achieved. Impressively, these results were achieved without the use of stents and with very early interventional equipment. Frequently, technical failure was not a function of an inability to cross the lesion with a wire but instead an inability to cross the lesion with the dilating balloon. New lower profile balloons make this problem very unusual because a recent report demonstrated technical failure of less than 10%,31 and a recent meta-analysis showed technical success rates of 94% for stenosis and of 64% for complete occlusions.32
Stents
The introduction of stents for treating arch vessel occlusive disease began in the 1990s, and they have become the primary mode of therapy for most surgeons treating this disease with an interventional approach.23,33–38 These studies reported technical success rates from 91% to 100%, with failures occurring only in patients with occlusions that could not be crossed. Follow-up in the larger of these series demonstrated patency rates of 77% to 100% over an 18- to 24-month period. It remains unclear whether primary stenting of all arch vessel lesions is clearly beneficial, and some authors have suggested that a selective approach to stenting may be a better alternative.39,40 The most common approach reported today involves the routine use of stents in treating these vessels, and it appears that when treating occlusions or complex stenoses, primary stenting should be the preferred approach. There is currently limited reported use of medicated stents for the treatment of supra-aortic trunk41 occlusive disease, and no medicated stents are either designed or approved by the Food and Drug Administration (FDA) for this condition.
Covered Stents
Covered stents for the treatment of arch vessel disease have been reported for aneurysm and traumatic injury,42–45 but no meaningful data are available to date on the use of these devices for the treatment of occlusive disease. These reports document excellent acute success of these devices in this setting, and patency of these devices has been maintained, although repeat interventions have been necessary at times for treatment of either kinks or angulation within the prosthesis. It may be that the use of these devices for occlusive disease may prove especially helpful in reducing the incidence of neointimal hyperplastic recurrent stenosis, but at present there is inadequate information to make a recommendation for their use in this setting.
Perioperative Medical Management
Antiplatelet Agents
There are few data regarding the use of antiplatelet therapies for intervention in the supra-aortic trunk. Much of what is recommended is based on inference from the use of these agents in patients with peripheral and cerebrovascular disease and their use in carotid bifurcation intervention (see Chapter 35). The benefit of the use of aspirin in patients with peripheral vascular disease is well documented, and it is recommended that all patients with peripheral or cerebrovascular disease be maintained on aspirin.46 In acute intervention, the routine use of aspirin is mandatory, and for interventions in the aortic arch, aspirin should be considered part of standard therapy. The use of clopidogrel for the treatment of patients with atherosclerotic vascular disease has also been well documented, and it provides a reduction in risk compared with aspirin use alone in this population.47 The combination of this agent with aspirin for the treatment of symptomatic carotid disease is beneficial in reducing embolic episodes, as documented by transcranial Doppler examinations,48 and the combination of aspirin and clopidogrel has shown benefit when used as the antiplatelet regimen in carotid stenting.49,50 The addition of glycoprotein IIb/IIIa inhibitors to the dual antiplatelet regimen of aspirin and clopidogrel has not been shown to reduce embolic events, and may significantly increase intracranial hemorrhagic complications.51–53 Based on this information, and with the proviso that the data are inferential, aspirin and clopidogrel therapy are recommended as the standard antiplatelet therapy for arch intervention.
Intraprocedural Heparin
Heparin is normally used as the intraoperative anticoagulant for intervention in the arch vessels, and at present, there are no meaningful data assessing alternative anticoagulant strategies in this clinical setting.54 The use of bivalirudin in carotid interventions has been reported,55 and this approach may prove to be a safe alternative to the routine use of unfractionated heparin, but without additional data, routine use of bivalirudin cannot be recommended. Currently, heparin administration adequate to maintain activated clotting times of greater than 250 to 300 seconds is recommended, and reversal of the heparin with protamine after intervention is reasonable.
Technical Details
After angiography of the arch, selective catheterization must be performed to gain adequate imaging of the arch vessels and allow performance of the intervention. In most instances, this is best achieved with a forward-facing catheter with minimal angulation. Options for this initial access include the vertebral (Cook, Inc., Bloomington, Indiana), Headhunter (Cook, Inc., Bloomington, Indiana), and angled Glide (Terumo, Tokyo, Japan) catheters. These less complex catheters allow simple access to the vessels, and if advancement of the catheter is necessary, it may be performed easily. When the angulation of the vessel origin mandates an increased angle on the catheter, alternatives include the C2 (Cook, Inc., Bloomington, Indiana), JR4 (Cordis, Mexico), and internal mammary (Boston Scientific, Mexico) catheters. The use of complex or reverse-curve catheters necessitates formation of the catheter within the aorta, which can increase embolic potential and may complicate access to these vessels. Nevertheless, use of these reverse-curve catheters may be essential because of the angulation of the vessel origin. Once selective access has been obtained, the vessel can be imaged, and intervention can be performed.
One advantage of intervention in this vascular distribution is the potential for an alternative access site for treatment. Brachial access for intervention in the subclavian arteries and the innominate artery can provide an approach to open an obstructive lesion that might otherwise prove impossible to treat from the femoral access route. These other access routes also provide alternative methods for imaging or insertion of protection devices while trying to intervene for treatment of stenotic lesions (Fig. 106-4).
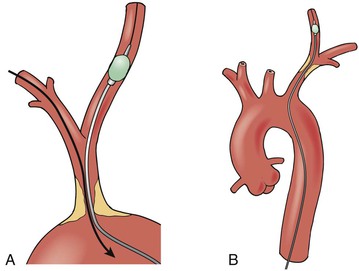
Figure 106-4 A, Left brachial or femoral access can provide a method to insert a protection device (shown here as a balloon) into the right common carotid artery during retrograde treatment of the innominate artery stenosis. B, Similarly, femoral access can allow protection of the vertebral artery during retrograde treatment of a left subclavian stenosis.
Left Subclavian Artery Intervention
Many of the techniques described here are applicable to interventions in the other vessels in this distribution and can be helpful to keep in one’s armamentarium. The other arch vessels less frequently undergo intervention because they are less often affected by occlusive disease. However, because of the increased angulation noted at the origin of these vessels, these interventions can prove to be more challenging, especially when performed from a groin approach.
Access to the left subclavian artery off the arch is usually the easiest of the three vessels to obtain. Often, only a mild angled catheter is required, and most interventions can be performed with a sheath rather than an angled guide catheter. Typically, after arch aortography with either a 4F or 5F flush catheter, an exchange is made for a selective catheter. After accessing the origin of the vessel, a 0.035-inch Glidewire (Terumo Cardiovascular Systems, Ann Arbor, Michigan) is passed across the lesion into the distal vessel. If a stiff Glidewire is used, it can be left in place, and a long 6F sheath can be advanced from the groin over this wire. If the Glidewire used has less support, wire exchange for either a Rosen (Infiniti Medical, Menlo Park, California) or Storq (Cordis, Bridgewater, New Jersey) wire can be performed, and the sheath then advanced over this wire. The sheath is positioned at the origin of the vessel and just below the disease. The vessel origin and the area of disease are positioned within the center of the imaging field to minimize distortion from parallax. Predilatation with a 4- to 6-mm angioplasty balloon allows determination of size and will ensure free passage of the stent. Frequently, this predilatation is performed with a balloon that is longer than the lesion itself to allow fixation of the balloon at either end of the stenosis. The balloon diameter chosen for predilatation should be slightly smaller than the vessel distally so that dissection of the distal vessel is not induced. This will limit the potential for damage to the origin of the vertebral artery. This technique also allows imaging of the distal extent of the lesion to gauge the involvement of the vertebral and internal mammary artery origins because preservation of these origins is critical, especially the vertebral artery origin. The length and diameter of the stent selected should be sufficient to ensure that the lesion is covered fully and the vessel is expanded to its true size and not that of a poststenotic dilated segment. The stent should not extend beyond the origin of the vertebral artery origin. Ideally, for lesions located proximally in the vessel, the stent should extend approximately 1 to 2 mm into the aortic lumen. If the balloon-expandable stent is undersized, it will allow further expansion by dilatation with a larger balloon. It is important to know the limits of expansion of the stent while still retaining the strut architecture, which provides adequate radial resistance to compression. After adequate dilatation of the stent in the vessel, if the proximal extent of the stent has been positioned partially in the aorta, it is helpful to “flare” this end of the stent by dilating it with an oversized balloon. This will further open the origin of the vessel and reduce protrusion of the stent into the aortic lumen, which can sometimes be an impediment to future interventions in the vascular system either in the arch vessels or in the coronary arteries.
In some situations, the interventionalist may choose to place the stent “protected” through the lesion. To do so, the sheath and dilator are passed through the lesion, and the sheath remains within the lesion. Typically, for origin disease of the vessel, a balloon-expandable stent is positioned just at the origin of the vessel, and if the stent has been placed in protected fashion, the sheath is withdrawn to expose the stent within the lesion. When using this approach, the interventionalist must ensure that the stent is positioned appropriately before complete withdrawal of the sheath. This positioning can sometimes be difficult to achieve, and thus, is not often used as the principal approach.
Vertebral Artery Protection
When the lesion approaches the origin of the vertebral artery and does not directly involve the origin of the subclavian artery, it is important to “protect” the vertebral artery origin. This can be done in two ways. The first is to use a slightly larger sheath and insert a second wire into the origin of the vertebral artery. If there is concern that the origin of the vessel may need to be partially covered, once the sheath is within the subclavian artery, the 0.035-inch wire can be exchanged for a 0.014-inch wire placed antegrade out the axillary artery. A second 0.014-inch wire can then be positioned in the vertebral artery. If a balloon-expandable stent is used in this region, it may be possible to place a 0.035-inch system over both wires and position the stent over both wires. This ensures that the origin of the vertebral artery will be protected. In this region, a self-expanding stent may be preferable because some vessel torsion and flexion occur outside the thorax. The delivery system of a self-expanding stent will not allow proximate placement to the vertebral origin if the delivery system is inserted over both wires. Therefore, the stent delivery system must be placed over the wire in the axillary artery. Placing this system on the wire in the vertebral artery poses a significant risk of dissecting the origin of the vertebral artery.
It is imperative in this situation to be able to adequately visualize the vertebral origin as the stent is being deployed, and for this reason, brachial access should be considered. Ultrasound guidance can be used to obtain micropuncture access to the brachial artery, which is cannulated in a retrograde direction with a 5F sheath. A straight catheter can be positioned in the proximal vessel just beyond the vertebral origin, and retrograde imaging via this catheter will allow precise visualization of the origin and positioning of the stent. An additional degree of safety can be afforded if a longer sheath (30-45 cm) is inserted into the brachial artery and wire access into the vertebral artery is obtained via this access. This will allow continuous angiographic imaging of the vertebral origin and protective wire access at the same time.
Embolic protection of the vertebral artery system has been described,56 but its routine use cannot be advocated because the risk of embolic problems with intervention in this region is very low, and the risks related to insertion of embolic protection devices remain undefined. Complex lesions proximal to the origin of the vertebral artery pose an increased risk; therefore, care should be taken when crossing and treating these lesions. In the setting of subclavian steal, it is clear that normalization of flow takes some time to occur, and there is inherent protection related to the reversed flow in the vertebral artery.57
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


