An occlusion or severe stenosis (angiographic culprit lesion) of the infarct-related artery is frequently located at the site of the maximum thrombus burden, whereas the origin of the plaque rupture (the true culprit) can be situated proximal or distal to it. The aim of this study was to examine stent coverage of true culprit lesions in 20 patients who underwent primary percutaneous coronary intervention and had Thrombolysis In Myocardial Infarction (TIMI) grade 3 flow restored in the infarct-related artery by angiographically guided direct stenting. Images of lesions were obtained using virtual histology–intravascular ultrasound before and after intervention (blinded to the operator). Plaque rupture sites were identified by intravascular ultrasound in 12 lesions (60%), 11 proximal and 1 distal to the minimum luminal area (MLA). Maximum necrotic core sites were found proximal to the MLA in 16 lesions, at the MLA in 3 lesions, and distal to the MLA in 1 lesion. Plaque rupture sites were fully covered by stents in 11 lesions. Virtual histology–intravascular ultrasound–derived thin-cap fibroatheroma longitudinal geographic misses were found in 10 lesions, 7 in the proximal reference segment and in 3 patients in the proximal and distal reference segments. In conclusion, in about 50% of patients who undergo primary percutaneous coronary intervention for ST-segment elevation myocardial infarction with optimal angiographic results, the stent does not fully cover the maximum necrotic core site related to the culprit lesion.
Most acute thrombotic coronary artery occlusions occur because of the rupture of an atherosclerotic plaque, including thin-cap fibroatheroma (TCFA). Postmortem studies have shown that the occlusion (the angiographic culprit lesion) is composed mainly of a fresh or organizing thrombus, while the plaque rupture, the main cause of acute coronary syndromes, is located proximal or distal to the angiographic culprit lesion and may not be lumen compromising. Therefore, when treating the angiographic culprit lesion with stent implantation, incomplete stent coverage of the true culprit lesion (missing the plaque rupture site) may occur when stent implantation is performed under angiographic guidance only. Radiofrequency or virtual histology (VH)–intravascular ultrasound (IVUS) is based on spectral analysis of ultrasound scatter and has shown good correlation with histopathology. This allows the detection of lipid, fibrous tissue, calcium, and necrotic core and thus the identification of TCFA. The purpose of the present study was to use IVUS and VH-IVUS to assess culprit lesion coverage when a coronary stent is implanted using standard angiographic guidance during primary percutaneous coronary intervention (PCI).
Methods
The present study was a single-center, prospective, observational registry. The study protocol was approved by the institutional review board of the Jagiellonian University College of Medicine in Krakow, Poland (KBET/63/B/2008) and conformed to the Declaration of Helsinki of 1996. All patients provided written informed consent before enrollment.
Patients aged >18 years with uncomplicated ST-segment elevation myocardial infarction (STEMI) <12 hours after symptom onset who underwent primary PCI were eligible. Patients were not eligible if angiography demonstrated left main coronary artery stenosis >50% or if the coronary anatomy of the culprit vessel was inappropriate for IVUS assessment or stent implantation. Culprit lesions were de novo and nonostial, without heavy calcification, located in the proximal or mid segment of the parent infarct-related artery, with reference vessel diameter ≥2.5 mm by visual estimation. IVUS pullback was conducted after Thrombolysis In Myocardial Infarction (TIMI) grade 3 flow was restored (either by spontaneous reperfusion or after thrombus aspiration) and before stent implantation. Stent length and diameter selection was based on angiography alone and was followed by direct stent implantation with postdilation as needed to achieve optimal angiographic results (residual angiographic diameter stenosis <20% and TIMI grade 3 flow). After finishing the procedure, IVUS pullback was repeated. Operators performing primary PCI were blinded to IVUS and VH-IVUS findings. Therefore, these findings did not influence primary PCI, which was carried out according to the standard practice of the center. IVUS was conducted using the commercially available Eagle Eye Gold phased-array IVUS probe (Volcano Corporation, Rancho Cordova, California). The probe was advanced >10 mm distal to the distal end of the angiographic culprit lesion, and motorized pullback up to the guiding catheter was performed at a speed of 0.5 mm/s using the nondisposable Volcano R100 pullback device. Radiofrequency backscatter data were collected simultaneously and triggered by the R-wave peak of the patient’s electrocardiogram using a dedicated IVUS console (Volcano Corporation). IVUS and VH-IVUS images were performed and archived for off-line analysis.
The region of interest was defined in each vessel as the stented lesion plus 10 mm proximal and distal to the edges of the stent, with the following exception. In 2 patients, in whom the lengths of TCFAs in the reference segment were >10 mm, the total length of the TCFA was measured. Each region of interest imaged by IVUS and VH-IVUS was analyzed by 2 different analysts to address interobserver and intraobserver variability.
IVUS analysis was performed according to the “American College of Cardiology Clinical Expert Consensus Document on Standards for Acquisition, Measurement and Reporting of Intravascular Ultrasound Studies (IVUS).” VH-IVUS analysis was performed according to “Tissue Characterisation Using Intravascular Radiofrequency Data Analysis: Recommendations for Acquisition, Analysis, Interpretation and Reporting.” The distances between maximum necrotic core and the plaque rupture and minimum luminal area (MLA) sites were measured.
Contour detection of the lumen and the media-adventitia interface was performed on every cross section and used to calculate volumes for the lesion and proximal and distal reference segments. The area between the external elastic membrane and the lumen-intima interface was defined as the plaque area and used to calculate plaque burden (plaque/external elastic membrane). Using VH-IVUS, plaque was classified and color-coded as necrotic core, fibrofatty, fibrotic, or calcific. A TCFA was defined as a confluent necrotic core of >10% of the plaque area, in direct contact with the lumen, with no evidence of a VH-IVUS-derived fibrous cap in 3 subsequent cross sections of the vessel. Plaque rupture was identified by IVUS as plaque ulceration or fissuring with a tear detected in a fibrous cap.
Off-line qualitative and quantitative coronary angiographic analysis was performed using QCAPlus (Sanders Data Systems, Palo Alto, California) by an experienced analyst (L.R.) blinded to the clinical data and procedural information. The IVUS analysis was performed using echoPlaque version 3 (Indec Medical Systems, Santa Clara, California). The VH-IVUS analysis was performed using pcVH version 2.2 and qVH (Volcano Corporation, Rancho Cordova, California) for tissue characterization and advanced analysis, respectively. IVUS and VH-IVUS data were analyzed by 2 independent analysts (J.J. and M.W.) blinded to the clinical data and procedural information, and all analyses were reviewed by single independent reviewer (J.L.). Overall, interobserver and intraobserver variability showed good intraclass correlations among analysts and for the same analyst at the core laboratory, with a higher level of agreement obtained for TCFA detection for intraobserver analysis and a lower level of agreement for interobserver analysis (κ = 0.933 and κ = 0.894, respectively).
The following software was used for statistical analysis: Freq, Npar1way, Univariate and Logistic; SAS version 9.2 (SAS Institute Inc., Cary, North Carolina). Discrete variables are presented as counts and percentages. Continuous variables are presented as mean ± SD. Differences were compared using chi-square tests. The value of p <0.05 was defined as statistically significant.
Results
Among 30 patients screened, 20 were enrolled in the study in compliance with the inclusion and exclusion criteria. Main reasons for screening failures were vessel tortuosity, excessive calcifications, and subtotal vessel stenoses that did not allow crossing with an IVUS catheter. Procedural data and off-line qualitative and quantitative coronary angiographic results are listed in Table 1 . Final TIMI grade 3 flow was achieved in 18 patients (90%). No deaths, reinfarctions, or repeat interventions were reported in hospital, at 30 days, or at 1 year in these 20 patients.
| Variable | Value |
|---|---|
| Infarct-related coronary artery | |
| Left anterior descending | 6 (30%) |
| Diagonal | 1 (5%) |
| Left circumflex | 2 (10%) |
| Right | 11 (55%) |
| Procedural details | |
| Thrombus aspiration due to baseline TIMI flow grade <3 | 12 (60%) |
| Abciximab | 11 (55%) |
| Direct stenting | 20 (100%) |
| Stent postdilatation | 14 (70%) |
| Bare-metal stent | 19 (95%) |
| Drug-eluting stent | 1 (5%) |
| Stent length (mm) | 15.7 ± 5.18 |
| Stent diameter (mm) | 3.35 ± 0.60 |
| Maximal balloon diameter (mm) | 3.64 ± 0.63 |
| Maximal inflation pressure (atm) | 14.3 ± 2.05 |
| Quantitative coronary angiography before stent implantation | |
| Lesion length (mm) | 14.27 ± 3.81 |
| Reference luminal diameter (mm) | 3.02 ± 0.63 |
| Minimal luminal diameter (mm) | 0.45 ± 0.52 |
| % diameter stenosis | 88 ± 23 |
| Quantitative coronary angiography after PCI | |
| Reference luminal diameter (mm) | 3.18 ± 0.51 |
| Minimal luminal diameter (mm) | 2.74 ± 0.48 |
| % diameter stenosis | 13 ± 5 |
| Stent length to lesion length ratio | 1.07 ± 0.19 |
| Ratio of stent diameter to reference luminal diameter before stent implantation | 1.11 ± 0.10 |
| Ratio of stent diameter to reference luminal diameter after PCI | 1.05 ± 0.10 |
| Ratio of maximal balloon diameter to reference luminal diameter before stent implantation | 1.21 ± 0.19 |
| Ratio of maximal balloon diameter to reference luminal diameter after PCI | 1.13 ± 0.11 |
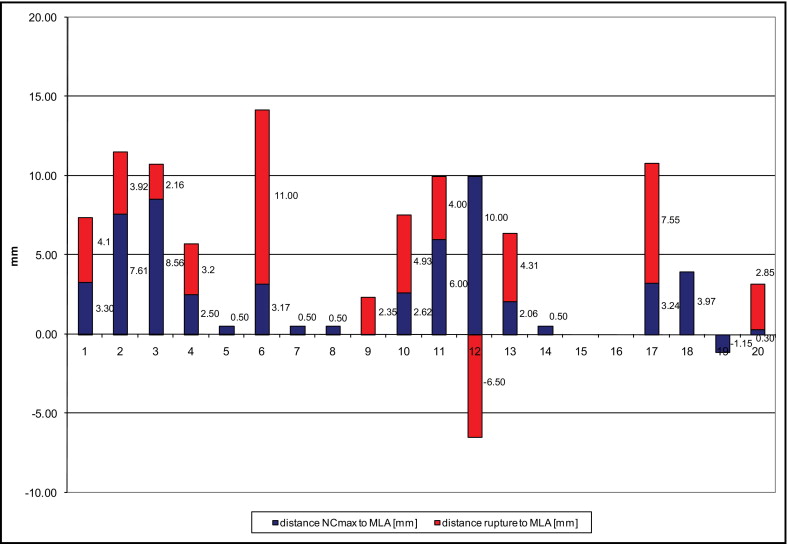
Plaque ruptures were identified in 12 lesions (60%), 11 proximal and 1 distal to the MLA site. The maximum necrotic core sites were proximal to the MLA sites in 16 lesions (80%) and distal to the MLA site in 1 lesion (5%). Plaque burden was significantly larger at the MLA site in comparison to the site of maximum necrotic core and to the plaque rupture site (82 ± 5% vs 74 ± 9% and 72 ± 9%, respectively, p = 0.003). Luminal diameter was significantly smaller at the MLA site in comparison to the site of maximum necrotic core and to the plaque rupture site (1.6 ± 0.2, 2.1 ± 0.3, and 2.2 ± 0.3 mm 2 , respectively, p = 0.002).
The distance between the plaque rupture site and the MLA site was 4.13 ± 1.70 mm (maximum 11.5 mm). The distance from the maximum necrotic core to the MLA site was 4.24 ± 3.42 mm (maximum 10.5 mm). The distribution of distances from the plaque rupture site and the maximum necrotic core site to the MLA site for individual patients is shown in Figure 1 . The distance between the MLA and the plaque rupture sites was less than the distance between the MLA and maximum necrotic core sites.

Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


