Viral Pulmonary Infections
GENERAL PRINCIPLES
• Viral respiratory infections account for nearly 50% of all acute respiratory illnesses. Most infections are self-limited.
• Approximately 200 antigenically distinct viruses cause multiple clinical syndromes ranging from common cold, pharyngitis, croup (i.e., laryngotracheobronchitis), tracheitis, bronchitis, bronchiolitis, and pneumonia.
• This chapter will introduce the major respiratory viruses encountered in clinical practice, assist in differentiating viral and bacterial respiratory infections, and guide antiviral therapy where specific therapy exists (Tables 16-1, 16-2, and 16-3).
Classification in the Normal Host
Upper Respiratory Tract Infections
• Rhinosinusitis is an upper respiratory tract infection (URTI) defined as inflammation of the mucosa of the nasal passage and paranasal sinuses lasting up to 4 weeks.
 The most common etiology is viral. Bacteria can secondarily infect an inflamed sinus cavity, but this only accounts for 0.5–2% of cases.1
The most common etiology is viral. Bacteria can secondarily infect an inflamed sinus cavity, but this only accounts for 0.5–2% of cases.1
 Since management of an acute viral rhinosinusitis (AVRS) is supportive, the main focus for the clinician should be in identifying those cases with acute bacterial rhinosinusitis (ABRS). Viral etiologies include rhinovirus, adenovirus, parainfluenza virus (PIV), influenza virus, human coronavirus (HCoV), and enterovirus.
Since management of an acute viral rhinosinusitis (AVRS) is supportive, the main focus for the clinician should be in identifying those cases with acute bacterial rhinosinusitis (ABRS). Viral etiologies include rhinovirus, adenovirus, parainfluenza virus (PIV), influenza virus, human coronavirus (HCoV), and enterovirus.
 The most common bacteria associated with ABRS are Streptococcus pneumoniae, Haemophilus influenzae, and Moraxella catarrhalis.
The most common bacteria associated with ABRS are Streptococcus pneumoniae, Haemophilus influenzae, and Moraxella catarrhalis.
 Significant complications of ABRS are rare and include orbital cellulitis, cavernous sinus thrombosis, osteomyelitis, meningitis, and brain abscess. These complications represent medical emergencies that require hospitalization.
Significant complications of ABRS are rare and include orbital cellulitis, cavernous sinus thrombosis, osteomyelitis, meningitis, and brain abscess. These complications represent medical emergencies that require hospitalization.
 The diagnosis is clinical. Acute rhinosinusitis of any etiology presents with three major symptoms: nasal congestion or blockage, purulent rhinorrhea, and facial pain or pressure.2,3
The diagnosis is clinical. Acute rhinosinusitis of any etiology presents with three major symptoms: nasal congestion or blockage, purulent rhinorrhea, and facial pain or pressure.2,3
 AVRS symptoms typically peak within 2–3 days of onset, decline gradually thereafter, and disappear within 10–14 days. Any pattern that deviates from the “classical” viral disease progression could suggest bacterial infection.
AVRS symptoms typically peak within 2–3 days of onset, decline gradually thereafter, and disappear within 10–14 days. Any pattern that deviates from the “classical” viral disease progression could suggest bacterial infection.
 Three criteria may help distinguish ABRS from AVRS4:
Three criteria may help distinguish ABRS from AVRS4:
 Persistent signs and symptoms lasting for ≥10 days.
Persistent signs and symptoms lasting for ≥10 days.
 Severe symptoms for 3–4 consecutive days at the beginning of illness: high fever (≥39°C) and purulent rhinorrhea or facial pain.
Severe symptoms for 3–4 consecutive days at the beginning of illness: high fever (≥39°C) and purulent rhinorrhea or facial pain.
 Double-sickening: new onset of fever or increased nasal discharge following a typical viral URTI that lasted 5–6 days and was initially improving.
Double-sickening: new onset of fever or increased nasal discharge following a typical viral URTI that lasted 5–6 days and was initially improving.
 Imaging studies such as plain radiographs and CT scans are of little diagnostic value in uncomplicated acute rhinosinusitis and are not routinely recommended. An abnormal radiographic finding cannot distinguish a viral from bacterial etiology.5
Imaging studies such as plain radiographs and CT scans are of little diagnostic value in uncomplicated acute rhinosinusitis and are not routinely recommended. An abnormal radiographic finding cannot distinguish a viral from bacterial etiology.5
 Cultures obtained by sinus aspiration are not indicated for uncomplicated ABRS. They could be performed if the patient has failed to respond to initial empiric antimicrobial therapy.
Cultures obtained by sinus aspiration are not indicated for uncomplicated ABRS. They could be performed if the patient has failed to respond to initial empiric antimicrobial therapy.
• Management of AVRS is supportive.
 Intranasal saline irrigation with either physiologic or hypertonic saline can be beneficial in symptomatic control, although the evidence supporting it is still very weak.4
Intranasal saline irrigation with either physiologic or hypertonic saline can be beneficial in symptomatic control, although the evidence supporting it is still very weak.4
 Topical or systemic decongestants, antihistamines, and mucolytics are frequently used for symptom control. However, there are no significant data to support their use. Topical decongestants should not be used for more than 3 consecutive days to avoid rebound congestion and tachyphylaxis.
Topical or systemic decongestants, antihistamines, and mucolytics are frequently used for symptom control. However, there are no significant data to support their use. Topical decongestants should not be used for more than 3 consecutive days to avoid rebound congestion and tachyphylaxis.
 Intranasal corticosteroids have been shown to provide a modest relief in symptoms when compared to placebo and should certainly be strongly considered in patients with allergic rhinitis.6
Intranasal corticosteroids have been shown to provide a modest relief in symptoms when compared to placebo and should certainly be strongly considered in patients with allergic rhinitis.6
 Antibiotics may be beneficial for patients with a clinical diagnosis of ABRS and who have severe/persistent symptoms, temperature ≥39°C, or double-sickening. Amoxicillin-clavulanate is the drug of choice and doxycycline is an alternative. The infectious Disease Society of America (IDSA) recommends against trimethoprim-sulfamethoxazole and macrolides. High-dose amoxicillin-clavulanate is recommended where there is a ≥10% endemic rate of invasive penicillin-non-susceptible S. pneumoniae, severe infection (e.g., temperature ≥39°C, systemic toxicity, threat of suppurative complication), daycare attendance, age <2 or >65, recent hospitalization, antibiotic use during the past month, and immunocompromised state.4
Antibiotics may be beneficial for patients with a clinical diagnosis of ABRS and who have severe/persistent symptoms, temperature ≥39°C, or double-sickening. Amoxicillin-clavulanate is the drug of choice and doxycycline is an alternative. The infectious Disease Society of America (IDSA) recommends against trimethoprim-sulfamethoxazole and macrolides. High-dose amoxicillin-clavulanate is recommended where there is a ≥10% endemic rate of invasive penicillin-non-susceptible S. pneumoniae, severe infection (e.g., temperature ≥39°C, systemic toxicity, threat of suppurative complication), daycare attendance, age <2 or >65, recent hospitalization, antibiotic use during the past month, and immunocompromised state.4
• Pharyngitis/tonsillitis
 The large majority of cases in adults are viral in etiology and do not require antibiotics. Antibiotics should be used in cases of Group A β-hemolytic streptococcal pharyngitis.
The large majority of cases in adults are viral in etiology and do not require antibiotics. Antibiotics should be used in cases of Group A β-hemolytic streptococcal pharyngitis.
 Symptoms strongly suggestive of a viral etiology include rhinorrhea, cough, oral ulcers, and hoarseness.7
Symptoms strongly suggestive of a viral etiology include rhinorrhea, cough, oral ulcers, and hoarseness.7
 The modified Centor criteria can be used to estimate the probability of streptococcal pharyngitis. One point each is given for the following: temperature >38°C, absence of cough, anterior cervical lymphadenopathy, and tonsillar swelling or exudate. The modification adds one point for age 3–14 and subtracts one point for age ≥45; age 15–44 has no effect on the score. The risk of streptococcal infection with ≤0 points is 1–2.5%; 1 point, 5–10%; 2 points, 11–17%; 3 points, 28–35%; and ≥4 points, 21–53%. 8–11
The modified Centor criteria can be used to estimate the probability of streptococcal pharyngitis. One point each is given for the following: temperature >38°C, absence of cough, anterior cervical lymphadenopathy, and tonsillar swelling or exudate. The modification adds one point for age 3–14 and subtracts one point for age ≥45; age 15–44 has no effect on the score. The risk of streptococcal infection with ≤0 points is 1–2.5%; 1 point, 5–10%; 2 points, 11–17%; 3 points, 28–35%; and ≥4 points, 21–53%. 8–11
 Regardless, the IDSA recommends rapid strep testing whenever there is consideration of streptococcal pharyngitis, as clinical features alone may cause under- and overdiagnosis.7
Regardless, the IDSA recommends rapid strep testing whenever there is consideration of streptococcal pharyngitis, as clinical features alone may cause under- and overdiagnosis.7
 Negative rapid strep tests should be confirmed with throat culture in children and adolescents due to a higher risk of developing acute rheumatic fever in these age groups. In adults, however, a backup culture is unnecessary because the pretest probability of rapid antigen detection testing is low to begin with.7
Negative rapid strep tests should be confirmed with throat culture in children and adolescents due to a higher risk of developing acute rheumatic fever in these age groups. In adults, however, a backup culture is unnecessary because the pretest probability of rapid antigen detection testing is low to begin with.7
 Common viral etiologies include rhinovirus, HCoV, adenovirus, herpes simplex virus (HSV), PIV, and influenza virus. Epstein–Barr virus (EBV), coxsackievirus, and acute HIV have also been identified.
Common viral etiologies include rhinovirus, HCoV, adenovirus, herpes simplex virus (HSV), PIV, and influenza virus. Epstein–Barr virus (EBV), coxsackievirus, and acute HIV have also been identified.
 Diagnostic testing of viral pharyngitis should be restricted to cases where symptoms fail to resolve within 1–2 weeks or for surveillance cultures during endemic virus outbreaks.
Diagnostic testing of viral pharyngitis should be restricted to cases where symptoms fail to resolve within 1–2 weeks or for surveillance cultures during endemic virus outbreaks.
 Treatment is mostly supportive with hydration, antipyretics, and analgesia. Topical anesthetics and lozenges alleviate throat pain.
Treatment is mostly supportive with hydration, antipyretics, and analgesia. Topical anesthetics and lozenges alleviate throat pain.
TABLE 16-1 COMMON RESPIRATORY VIRUS INFECTIONS
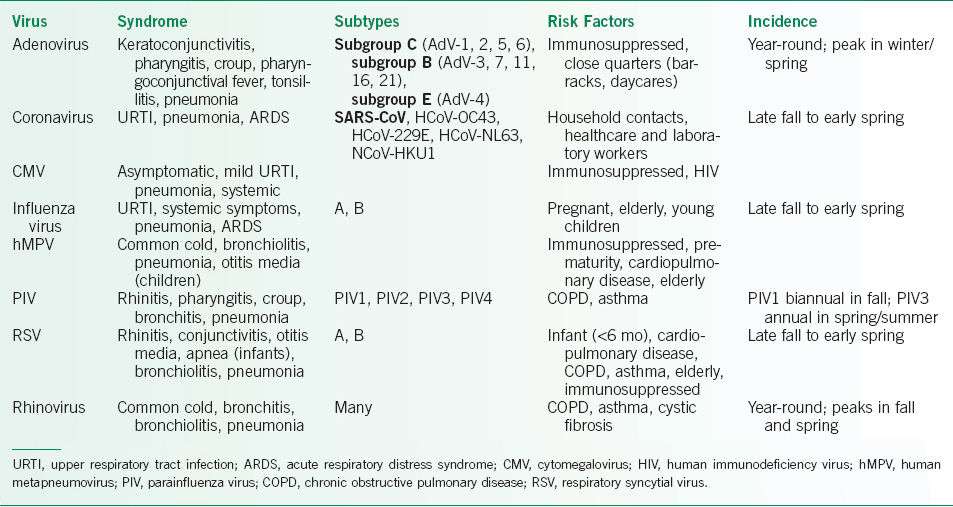
TABLE 16-2 DIAGNOSTIC STRATEGIES FOR VIRAL RESPIRATORY INFECTIONS
TABLE 16-3 TARGETED ANTIVIRAL THERAPIES FOR VIRAL RESPIRATORY PATHOGENS

Lower Respiratory Tract Infections
• Bronchitis
 Bronchitis is classified as acute, which is essentially always infectious, or chronic.
Bronchitis is classified as acute, which is essentially always infectious, or chronic.
 Chronic bronchitis is a nonspecific clinical term with several meanings and is usually not due to infection.
Chronic bronchitis is a nonspecific clinical term with several meanings and is usually not due to infection.
 In the general context of chronic obstructive pulmonary disease (COPD), it is defined as cough with sputum production for 3 months in each of the 2 prior years without other explanation—it may or may not be associated with demonstrable airflow obstruction on pulmonary function testing.
In the general context of chronic obstructive pulmonary disease (COPD), it is defined as cough with sputum production for 3 months in each of the 2 prior years without other explanation—it may or may not be associated with demonstrable airflow obstruction on pulmonary function testing.
 In an even broader context chronic bronchitis can imply a productive cough for >8–12 weeks. Chronic cough is discussed in detail in Chapter 8.
In an even broader context chronic bronchitis can imply a productive cough for >8–12 weeks. Chronic cough is discussed in detail in Chapter 8.
 Acute bronchitis is a self-limited infection with cough as the predominant symptom usually lasting up to 3 weeks but can be as long as 6 weeks.12 Significant rhinorrhea, fever, dyspnea, tachypnea, or hypoxemia suggests an alternative diagnosis.
Acute bronchitis is a self-limited infection with cough as the predominant symptom usually lasting up to 3 weeks but can be as long as 6 weeks.12 Significant rhinorrhea, fever, dyspnea, tachypnea, or hypoxemia suggests an alternative diagnosis.
 Ninety percent cases of acute bronchitis are viral in nature, most commonly PIV, influenza, adenovirus, HCoV, human metapneumovirus (hMPV), PIV, respiratory syncytial virus (RSV), and rhinovirus.13 When caused by bacteria, mostly the organisms are M. pneumoniae, C. pneumoniae, and B. pertussis. Superinfection with typical respiratory pathogens (i.e., S. pneumoniae, H. influenzae, and M. catarrhalis) is known to occur but are presumed to be very unusual.
Ninety percent cases of acute bronchitis are viral in nature, most commonly PIV, influenza, adenovirus, HCoV, human metapneumovirus (hMPV), PIV, respiratory syncytial virus (RSV), and rhinovirus.13 When caused by bacteria, mostly the organisms are M. pneumoniae, C. pneumoniae, and B. pertussis. Superinfection with typical respiratory pathogens (i.e., S. pneumoniae, H. influenzae, and M. catarrhalis) is known to occur but are presumed to be very unusual.
 Treatment is supportive with antitussives, expectorants, inhaled bronchodilators, and alternative therapies, despite a paucity of data to support their use.
Treatment is supportive with antitussives, expectorants, inhaled bronchodilators, and alternative therapies, despite a paucity of data to support their use.
 Routine use of antibiotics is discouraged.14–17 Likewise, there is limited value in treating smokers without COPD with antibiotics. An important exception is when B. pertussis is known or suspected in order to limit spread, particularly to unvaccinated infants. In this case, the treatment of choice is azithromycin. Unless started early, antibiotic treatment has little impact on the course of pertussis in typical, previously vaccinated adolescents and adults.12
Routine use of antibiotics is discouraged.14–17 Likewise, there is limited value in treating smokers without COPD with antibiotics. An important exception is when B. pertussis is known or suspected in order to limit spread, particularly to unvaccinated infants. In this case, the treatment of choice is azithromycin. Unless started early, antibiotic treatment has little impact on the course of pertussis in typical, previously vaccinated adolescents and adults.12
 Inhaled bronchodilators may be beneficial in patients who have airflow restriction and wheeze.18
Inhaled bronchodilators may be beneficial in patients who have airflow restriction and wheeze.18
 Bronchiolitis is an almost exclusively viral infection of the bronchioles and typically occurs in <2-year-old infants though it has been reported in adults.
Bronchiolitis is an almost exclusively viral infection of the bronchioles and typically occurs in <2-year-old infants though it has been reported in adults.
 Differentiated from bronchitis by respiratory symptoms, such as tachypnea and wheezing.
Differentiated from bronchitis by respiratory symptoms, such as tachypnea and wheezing.
 RSV is the most common etiology, but other viruses include rhinovirus, hMPV, and the more recently discovered human bocavirus (HBoV).
RSV is the most common etiology, but other viruses include rhinovirus, hMPV, and the more recently discovered human bocavirus (HBoV).
 Treatment is primarily supportive with supplemental oxygen. Continuous positive airway pressure and high-flow nasal cannula therapy may provide effective ventilatory support and decrease the need for intubation.21,22
Treatment is primarily supportive with supplemental oxygen. Continuous positive airway pressure and high-flow nasal cannula therapy may provide effective ventilatory support and decrease the need for intubation.21,22
 Several studies have evaluated the effectiveness of inhaled bronchodilators (epinephrine and albuterol), systemic corticosteroids, inhaled hypertonic saline, and heliox. None of these therapies demonstrated a consistent benefit on mortality or length of hospitalization.23
Several studies have evaluated the effectiveness of inhaled bronchodilators (epinephrine and albuterol), systemic corticosteroids, inhaled hypertonic saline, and heliox. None of these therapies demonstrated a consistent benefit on mortality or length of hospitalization.23
• Pneumonia24
 Fifty percent to 70% of childhood pneumonia and up to 30% of adult pneumonia cases have been attributed to a viral etiology. Likely organisms include RSV, rhinovirus, influenza virus, hMPV, PIV, HBoV, and adenovirus.
Fifty percent to 70% of childhood pneumonia and up to 30% of adult pneumonia cases have been attributed to a viral etiology. Likely organisms include RSV, rhinovirus, influenza virus, hMPV, PIV, HBoV, and adenovirus.
 Symptoms typically include fever, tachypnea, tachycardia, and clinical findings of lung involvement on examination. Other symptoms may include cough, rhinorrhea, sinus congestion, chills, and myalgias.
Symptoms typically include fever, tachypnea, tachycardia, and clinical findings of lung involvement on examination. Other symptoms may include cough, rhinorrhea, sinus congestion, chills, and myalgias.
 Physical examination and CXR may demonstrate consolidation due to alveolar or interstitial infiltrates in a lobar or multilobar distribution.
Physical examination and CXR may demonstrate consolidation due to alveolar or interstitial infiltrates in a lobar or multilobar distribution.
 Viral pneumonia may be complicated by secondary bacterial pneumonia or concurrent viral and bacterial infection.
Viral pneumonia may be complicated by secondary bacterial pneumonia or concurrent viral and bacterial infection.
 No specific clinical findings clearly differentiate viral from bacterial pneumonia. A high fever (>38.5°C), high respiratory rate, lobar consolidation on chest radiography and significantly elevated levels of C-reactive protein (CRP), white blood cell (WBC) count, and procalcitonin (PCT) suggest bacterial etiology.
No specific clinical findings clearly differentiate viral from bacterial pneumonia. A high fever (>38.5°C), high respiratory rate, lobar consolidation on chest radiography and significantly elevated levels of C-reactive protein (CRP), white blood cell (WBC) count, and procalcitonin (PCT) suggest bacterial etiology.
 Mild cases improve with supportive management on an outpatient basis, while severe cases may necessitate admission to an intensive care unit (ICU), respiratory support with mechanical ventilation or other aggressive measures.
Mild cases improve with supportive management on an outpatient basis, while severe cases may necessitate admission to an intensive care unit (ICU), respiratory support with mechanical ventilation or other aggressive measures.
 Empiric antibiotics should be given in cases of severe pneumonia while awaiting culture results. Once confirmatory diagnostic testing is completed, therapy should be narrowed to target the pathogen.25
Empiric antibiotics should be given in cases of severe pneumonia while awaiting culture results. Once confirmatory diagnostic testing is completed, therapy should be narrowed to target the pathogen.25
 Specific antiviral therapies are discussed in more detail below.
Specific antiviral therapies are discussed in more detail below.
 Systemic corticosteroid use for viral pneumonia is controversial and effects may vary depending on the particular viral etiology.
Systemic corticosteroid use for viral pneumonia is controversial and effects may vary depending on the particular viral etiology.
Respiratory Virus Infections in Chronic Airways Disease
• Viral lower respiratory tract infections in infants have been linked to later development of asthma in childhood, especially RSV and rhinovirus.
• Viral respiratory infections can lead to exacerbations of asthma and COPD and more severe respiratory infections and prolonged disease courses in these patients.
 RSV, influenza virus, rhinovirus, and parainfluenza are common culprits.
RSV, influenza virus, rhinovirus, and parainfluenza are common culprits.
 Patients with acute COPD exacerbation are more likely to have viruses detected in airway samples than those with stable COPD.
Patients with acute COPD exacerbation are more likely to have viruses detected in airway samples than those with stable COPD.
• Influenza vaccinations of patients with asthma/COPD are recommended and may decrease the risk of exacerbations in some patients.
Respiratory Virus Infections in Special Adult Populations
• Pregnancy26
 Susceptibility to virus infections is unchanged during pregnancy. However, the severity of viral respiratory infections can be worse in pregnancy, especially during the second and third trimesters.
Susceptibility to virus infections is unchanged during pregnancy. However, the severity of viral respiratory infections can be worse in pregnancy, especially during the second and third trimesters.
 Treatment is supportive with hydration, antipyretics, oxygen, blood pressure, and ventilatory support. Specific antiviral therapy should be used if available.
Treatment is supportive with hydration, antipyretics, oxygen, blood pressure, and ventilatory support. Specific antiviral therapy should be used if available.
 Antipyretics help to prevent fetal tachycardia and congenital abnormalities related to high maternal fevers.
Antipyretics help to prevent fetal tachycardia and congenital abnormalities related to high maternal fevers.
 Acyclovir is classified as category B but is recommended only for cases of serious infection and not for routine use in pregnancy.
Acyclovir is classified as category B but is recommended only for cases of serious infection and not for routine use in pregnancy.
 Ribavirin is a teratogen and is contraindicated in pregnancy.
Ribavirin is a teratogen and is contraindicated in pregnancy.
 Controlled data on the safety of many other antiviral compounds in pregnancy are lacking. Most (oseltamivir, zanamivir, ganciclovir, cidofovir) are classified as category C by the U.S. Food and Drug Administration (FDA) and should be used only when the benefits of therapy outweigh the risks.
Controlled data on the safety of many other antiviral compounds in pregnancy are lacking. Most (oseltamivir, zanamivir, ganciclovir, cidofovir) are classified as category C by the U.S. Food and Drug Administration (FDA) and should be used only when the benefits of therapy outweigh the risks.
 Killed or inactivated vaccines have been shown to be safe in pregnancy, especially during seasonal outbreaks when benefits outweigh small risks. All pregnant females should be offered influenza vaccination.
Killed or inactivated vaccines have been shown to be safe in pregnancy, especially during seasonal outbreaks when benefits outweigh small risks. All pregnant females should be offered influenza vaccination.
• Immunocompromised
 Solid organ transplant recipients are at increased risk for developing cytomegalovirus (CMV), HSV-1, EBV, and varicella zoster virus (VZV), and more recently hMPV respiratory infections, especially in setting of prolonged respiratory failure in the ICU.
Solid organ transplant recipients are at increased risk for developing cytomegalovirus (CMV), HSV-1, EBV, and varicella zoster virus (VZV), and more recently hMPV respiratory infections, especially in setting of prolonged respiratory failure in the ICU.
 Adenovirus has also been documented in transplant recipients and may occur as a result of reactivation of latent virus.
Adenovirus has also been documented in transplant recipients and may occur as a result of reactivation of latent virus.
Prevention
• Vaccination, when available, remains the best means of preventing viral respiratory illness, but few vaccines are available.
• Influenza vaccine
 Vaccine strains may change yearly, so influenza vaccines must be administered annually.
Vaccine strains may change yearly, so influenza vaccines must be administered annually.
 Influenza virus vaccines are manufactured as an inactivated (killed virus) vaccine injection or as a live attenuated vaccine nasal spray.
Influenza virus vaccines are manufactured as an inactivated (killed virus) vaccine injection or as a live attenuated vaccine nasal spray.
 High-dose influenza vaccination may be considered in adults ≥65 years old, though this is not expressly recommended by Centers for Disease Control (CDC) Advisory Committee on Immunization Practices.27
High-dose influenza vaccination may be considered in adults ≥65 years old, though this is not expressly recommended by Centers for Disease Control (CDC) Advisory Committee on Immunization Practices.27
• Varicella Zoster Virus vaccine
 A live, attenuated vaccine given in two doses. Dose #1 is generally administered to infants between 12 and 15 months. Dose #2 is given to children at 4–6 years.
A live, attenuated vaccine given in two doses. Dose #1 is generally administered to infants between 12 and 15 months. Dose #2 is given to children at 4–6 years.
 In adults the vaccine is administered as a 2-dose regimen 4–8 weeks apart.
In adults the vaccine is administered as a 2-dose regimen 4–8 weeks apart.
 Contraindicated in pregnant women, immunosuppressed patients, and patients who have other active illnesses.
Contraindicated in pregnant women, immunosuppressed patients, and patients who have other active illnesses.
• All vaccines are contraindicated in patients who are allergic to specific vaccine components (e.g., eggs in the case of most influenza virus vaccines) or who have had a severe allergic reaction to a previous dose of the particular vaccine. Flublok is a recently approved egg-free, trivalent, recombinant hemagglutinin (HA), injectable influenza vaccine.
• Additional information regarding specific vaccines is available on the CDC website, www.cdc.gov/vaccines. Accessed 9/10/15.
Diagnosis
• Diagnosis may be possible, based on clinical grounds alone and particularly during seasonal outbreaks (Table 16-2).
• Radiographic findings are not pathognomonic for specific viral etiologies.
• Diagnostic specimens can be collected from nasopharyngeal or oral swabs, nasal washings, induced sputum, nasopharyngeal or tracheal aspirates, bronchial washings, bronchoalveolar lavage (BAL), endobronchial brush biopsy, or lung biopsy (transbronchial vs. percutaneous needle biopsy), depending on the examination findings and severity of disease.
 In children, nasopharyngeal swabs and washings have similar sensitivities for detection of respiratory viruses by polymerase chain reaction (PCR).
In children, nasopharyngeal swabs and washings have similar sensitivities for detection of respiratory viruses by polymerase chain reaction (PCR).
 In adults, nasopharyngeal swab is more sensitive than throat swab.
In adults, nasopharyngeal swab is more sensitive than throat swab.
• Viral culture remains the gold standard for diagnosing many viral infections. However, prolonged incubation times required to grow the virus in the laboratory preclude the utility of viral culture in many cases.
• Multiplex PCR testing is becoming more common, due to its high sensitivity, specificity, and rapidity.
• Direct and indirect fluorescence antibody-based assays and enzyme-linked immunosorbent assays (ELISA) are also useful for detection of certain viral pathogens.
• Cytopathology may also aid diagnosis by demonstrating cytopathologic effect (adenovirus), giant cells (CMV) or syncytial formation (RSV) in tissue specimens.
Treatment
Supportive Care
• Supportive care with adequate hydration and symptom control are mainstays of treatment.
 Nasal steroids, antipyretics, antihistamines, decongestants, analgesics, and bronchodilators are sometimes helpful in appropriate patients.
Nasal steroids, antipyretics, antihistamines, decongestants, analgesics, and bronchodilators are sometimes helpful in appropriate patients.
 Steroids may be useful for significant wheezing.
Steroids may be useful for significant wheezing.
 Nebulized epinephrine can be used in cases of stridor.
Nebulized epinephrine can be used in cases of stridor.
• In severe cases, transfer to an ICU and ventilatory support with noninvasive or invasive mechanical ventilation may be necessary.
Specific Antiviral Therapies
• Targeted antiviral therapies exist for some viral respiratory illnesses such as influenza, CMV, HSV, RSV, adenovirus, and severe acute respiratory syndrome coronavirus (SARS-CoV). Others are currently in development.
• These drugs target specific viral proteins including DNA polymerases, proteases, ion channels, and neuraminidase (NA) (in the case of influenza virus).
• Newer therapies such as palivizumab are monoclonal antibodies directed against viral proteins (RSV).
• Table 16-3 lists common viral respiratory pathogens, directed antiviral therapies and vaccines, if available.
SPECIAL CONSIDERATIONS: SPECIFIC VIRAL PATHOGENS
Adenovirus
• Virology: Adenovirus is a nonenveloped, double-stranded DNA virus. There are 51 different serotypes with 6 subgroups, A through F. Adenoviruses of subgroup C primarily infect the upper respiratory tract while viruses in subgroups B and E cause disease of the lower respiratory tract. They can integrate into the host genome leading to latent infection.28
• Epidemiology
 Adenoviruses have a worldwide distribution, and infections are more frequent in winter and spring.
Adenoviruses have a worldwide distribution, and infections are more frequent in winter and spring.
 Up to 5% of acute respiratory infections in children are attributed to adenoviruses so that by age 10 most individuals have serologic evidence of adenoviral infection.
Up to 5% of acute respiratory infections in children are attributed to adenoviruses so that by age 10 most individuals have serologic evidence of adenoviral infection.
 Adenovirus is commonly encountered in households and daycare centers where young children are found.
Adenovirus is commonly encountered in households and daycare centers where young children are found.
 Epidemics of adenovirus acute respiratory disease (ARD) have been reported among military recruits, immunocompromised patients, and in daycare settings.
Epidemics of adenovirus acute respiratory disease (ARD) have been reported among military recruits, immunocompromised patients, and in daycare settings.
 Transmission occurs via fomites, aerosolized particles, and the fecal–oral route. Adenoviruses can cause persistent infections, and the virus may be shed in the feces for months.
Transmission occurs via fomites, aerosolized particles, and the fecal–oral route. Adenoviruses can cause persistent infections, and the virus may be shed in the feces for months.
 Adenoviral infections have been transmitted to kidney and liver transplant recipients, suggesting that reactivation of latent virus (possibly in the transplanted organ) may be another important mode of transmission.
Adenoviral infections have been transmitted to kidney and liver transplant recipients, suggesting that reactivation of latent virus (possibly in the transplanted organ) may be another important mode of transmission.
 Vertical transmission has been reported in infants who were exposed to infected cervical secretions.
Vertical transmission has been reported in infants who were exposed to infected cervical secretions.
• Clinical presentation
 The presentation depends on the age and immune status of the infected host.
The presentation depends on the age and immune status of the infected host.
 Adenovirus can cause upper respiratory tract illnesses such as coryza, pharyngitis, and croup but can also cause lower respiratory tract disease—that is, laryngotracheobronchitis, bronchiolitis, and pneumonia.
Adenovirus can cause upper respiratory tract illnesses such as coryza, pharyngitis, and croup but can also cause lower respiratory tract disease—that is, laryngotracheobronchitis, bronchiolitis, and pneumonia.
 Pharyngoconjunctival fever is an adenovirus syndrome presenting with pharyngitis, conjunctival injection, fever, and cervical lymphadenopathy.
Pharyngoconjunctival fever is an adenovirus syndrome presenting with pharyngitis, conjunctival injection, fever, and cervical lymphadenopathy.
 Keratoconjunctivitis presenting as pink eye without purulent discharge is caused by adenovirus serotypes 8, 19, and 37.
Keratoconjunctivitis presenting as pink eye without purulent discharge is caused by adenovirus serotypes 8, 19, and 37.
 Gastrointestinal (GI) symptoms are caused by other adenovirus serotypes.
Gastrointestinal (GI) symptoms are caused by other adenovirus serotypes.
 Adenovirus is the most common cause of tonsillitis in infants. Exudative tonsillitis and palpable cervical adenopathy may be seen, making differentiation from strep throat in older children difficult.
Adenovirus is the most common cause of tonsillitis in infants. Exudative tonsillitis and palpable cervical adenopathy may be seen, making differentiation from strep throat in older children difficult.
 Pneumonia is most common in infants but rare in immunocompetent adults.
Pneumonia is most common in infants but rare in immunocompetent adults.
 Complications include bronchiectasis in children and ARD in young adults. ARD is especially common in close-quarter dwellings. Patients with ARD develop fever, pharyngitis, cough, hoarseness, and conjunctivitis.
Complications include bronchiectasis in children and ARD in young adults. ARD is especially common in close-quarter dwellings. Patients with ARD develop fever, pharyngitis, cough, hoarseness, and conjunctivitis.
 Bone marrow transplant patients may develop a wide range of respiratory clinical syndromes, including pneumonia. Solid organ transplant recipients may develop asymptomatic shedding, all manner of respiratory syndromes, and even fatal disseminated disease. Adenoviral pneumonia is a well-known early complication of lung transplantation.
Bone marrow transplant patients may develop a wide range of respiratory clinical syndromes, including pneumonia. Solid organ transplant recipients may develop asymptomatic shedding, all manner of respiratory syndromes, and even fatal disseminated disease. Adenoviral pneumonia is a well-known early complication of lung transplantation.
• Diagnosis
 The diagnosis is difficult to make on clinical grounds alone. PCR-based assays are the test of choice.
The diagnosis is difficult to make on clinical grounds alone. PCR-based assays are the test of choice.
 Viral culture is the historical gold standard. All adenoviruses except serotypes 40 and 41 cause a characteristic cytopathic effect in culture. Samples for culture can be obtained from nasopharyngeal swabs or aspirates, throat washings or swabs, rectal swabs, urine, CSF, or tissue biopsies. Cultures may take up to a week for completion and may not detect adenovirus in cases where there may be a low viral load (e.g., immunocompromised hosts). Because prolonged shedding may be seen in immunocompromised patients without overt disease, culture positivity should be interpreted with respect to the clinical situation.
Viral culture is the historical gold standard. All adenoviruses except serotypes 40 and 41 cause a characteristic cytopathic effect in culture. Samples for culture can be obtained from nasopharyngeal swabs or aspirates, throat washings or swabs, rectal swabs, urine, CSF, or tissue biopsies. Cultures may take up to a week for completion and may not detect adenovirus in cases where there may be a low viral load (e.g., immunocompromised hosts). Because prolonged shedding may be seen in immunocompromised patients without overt disease, culture positivity should be interpreted with respect to the clinical situation.
 Histopathology may provide definitive diagnosis of adenovirus in tissue biopsies and can be supplemented by other detection techniques (PCR, immunohistochemistry).
Histopathology may provide definitive diagnosis of adenovirus in tissue biopsies and can be supplemented by other detection techniques (PCR, immunohistochemistry).
• Treatment
 Treatment is supportive.
Treatment is supportive.
 In case reports and small series of solid organ transplant and bone marrow transplant patients, cidofovir has shown the most promise in treating adenoviral infections, especially when therapy is initiated early.28
In case reports and small series of solid organ transplant and bone marrow transplant patients, cidofovir has shown the most promise in treating adenoviral infections, especially when therapy is initiated early.28
 Randomized, controlled trials of antiviral agents are presently lacking, and data on other antiviral agents including ribavirin and vidarabine are conflicting.
Randomized, controlled trials of antiviral agents are presently lacking, and data on other antiviral agents including ribavirin and vidarabine are conflicting.
Coronavirus and SARS Virus
• Virology
 HCoV are positive-sense, single-stranded RNA viruses encased in a crown-like envelope with a diameter of 80–160 nm.
HCoV are positive-sense, single-stranded RNA viruses encased in a crown-like envelope with a diameter of 80–160 nm.
 HCoV-OC43, HCoV-229E, and the recently identified HCoV-NL63 and HCoV-HKU1 strains cause community-acquired respiratory infections.
HCoV-OC43, HCoV-229E, and the recently identified HCoV-NL63 and HCoV-HKU1 strains cause community-acquired respiratory infections.
 HCoV also include SARS-CoV, the causative agent of the SARS epidemic.
HCoV also include SARS-CoV, the causative agent of the SARS epidemic.
 Primary sites of replication include the lungs and intestinal tract.
Primary sites of replication include the lungs and intestinal tract.
• Epidemiology
 HCoV were initially discovered in the 1960s and are responsible for 10–20% of cases of the common cold.
HCoV were initially discovered in the 1960s and are responsible for 10–20% of cases of the common cold.
 Respiratory infections are spread in a manner similar to that of rhinoviruses, via direct contact with infected secretions or via large aerosol droplets.
Respiratory infections are spread in a manner similar to that of rhinoviruses, via direct contact with infected secretions or via large aerosol droplets.
 In temperate climates, they cause disease in late fall, winter, and early spring and are associated with outbreaks every 2–4 years.
In temperate climates, they cause disease in late fall, winter, and early spring and are associated with outbreaks every 2–4 years.
 In late 2002 and into 2003, an outbreak of SARS-CoV originated in China and Hong Kong and then spread globally causing more than 8000 cases with a case fatality rate of 9.6%.29 China, Taiwan, Hong Kong, Singapore, and Canada experienced the highest number of cases. Initial human cases of SARS-CoV appeared to be acquired from infected civets, although more recent data suggest that bats may be the natural reservoir for the virus. Those at increased risk include wildlife handlers, household contacts, healthcare workers, and laboratory workers.30
In late 2002 and into 2003, an outbreak of SARS-CoV originated in China and Hong Kong and then spread globally causing more than 8000 cases with a case fatality rate of 9.6%.29 China, Taiwan, Hong Kong, Singapore, and Canada experienced the highest number of cases. Initial human cases of SARS-CoV appeared to be acquired from infected civets, although more recent data suggest that bats may be the natural reservoir for the virus. Those at increased risk include wildlife handlers, household contacts, healthcare workers, and laboratory workers.30
 HCoV are stable at room temperature for 7 days but can be inactivated by common hospital disinfectants.
HCoV are stable at room temperature for 7 days but can be inactivated by common hospital disinfectants.
• Clinical presentation
 In adults, HCoV causes an acute URTI that is very similar to rhinovirus infection. HCoV are also implicated as important causes of acute otitis media in children and triggers of asthma exacerbations.
In adults, HCoV causes an acute URTI that is very similar to rhinovirus infection. HCoV are also implicated as important causes of acute otitis media in children and triggers of asthma exacerbations.
 Newer strains have been shown to cause LRTI, including severe pneumonia. Much of the pathogenesis is believed to derive from a significant host response to infection rather than from direct damage by the virus itself.
Newer strains have been shown to cause LRTI, including severe pneumonia. Much of the pathogenesis is believed to derive from a significant host response to infection rather than from direct damage by the virus itself.
 Potential complications include secondary bacterial infection, acute respiratory distress syndrome (ARDS), and acute respiratory failure requiring mechanical ventilation.
Potential complications include secondary bacterial infection, acute respiratory distress syndrome (ARDS), and acute respiratory failure requiring mechanical ventilation.
• Diagnosis
 RT-PCR is the standard diagnostic test for HCoV.
RT-PCR is the standard diagnostic test for HCoV.
 Other diagnostic tests include serologic detection and antigen detection but these are less useful clinically.
Other diagnostic tests include serologic detection and antigen detection but these are less useful clinically.
 For SARS-CoV, the diagnosis should be considered in patients at risk for SARS exposure (wildlife handlers from Southeast Asia, close family contacts, healthcare workers, and laboratory personnel) who present with a rapidly progressive pneumonia.
For SARS-CoV, the diagnosis should be considered in patients at risk for SARS exposure (wildlife handlers from Southeast Asia, close family contacts, healthcare workers, and laboratory personnel) who present with a rapidly progressive pneumonia.
• Treatment
 Patients with suspected HCoV, including SARS, should be placed on contact, droplet, and airborne precautions. This is especially important as up to one-third of SARS cases occurred in healthcare workers.
Patients with suspected HCoV, including SARS, should be placed on contact, droplet, and airborne precautions. This is especially important as up to one-third of SARS cases occurred in healthcare workers.
 Treatment is primarily supportive with symptom management, hydration, and ventilatory support. One study showed that early noninvasive ventilation in patients with severe SARS-CoV disease reduced the need for intubation and improved mortality.
Treatment is primarily supportive with symptom management, hydration, and ventilatory support. One study showed that early noninvasive ventilation in patients with severe SARS-CoV disease reduced the need for intubation and improved mortality.
 There are no randomized controlled trials of antiviral therapies in HCoV infection. Data from retrospective studies and case series during the SARS epidemic are inconclusive in regard to effective therapies.31
There are no randomized controlled trials of antiviral therapies in HCoV infection. Data from retrospective studies and case series during the SARS epidemic are inconclusive in regard to effective therapies.31
 Promising treatments have included viral protease inhibitors (lopinavir/ritonavir or nelfinavir), interferon, and convalescent plasma. NO may also improve outcome in severe respiratory failure.
Promising treatments have included viral protease inhibitors (lopinavir/ritonavir or nelfinavir), interferon, and convalescent plasma. NO may also improve outcome in severe respiratory failure.
 Combination therapy with ribavirin and/or corticosteroids may also be beneficial. Further studies are needed for confirmation.
Combination therapy with ribavirin and/or corticosteroids may also be beneficial. Further studies are needed for confirmation.
 Newer directed therapies including monoclonal antibodies targeting the SARS-CoV S protein or its receptor, angiotensin-converting enzyme 2 (ACE2) are in development.
Newer directed therapies including monoclonal antibodies targeting the SARS-CoV S protein or its receptor, angiotensin-converting enzyme 2 (ACE2) are in development.
Cytomegalovirus
• Virology: CMV, a member of the Herpesviridae family, is a large, enveloped double-stranded DNA virus with a diameter of 120–200 nm.
• Epidemiology
 Active CMV is often found in immunocompromised patients. It can affect multiple organ systems but the lung, GI tract, and kidney are most common.
Active CMV is often found in immunocompromised patients. It can affect multiple organ systems but the lung, GI tract, and kidney are most common.
 CMV can also be a source of severe viral pneumonia, even in immunocompetent individuals.
CMV can also be a source of severe viral pneumonia, even in immunocompetent individuals.
 Seroprevalence increases with age and number of sexual partners.
Seroprevalence increases with age and number of sexual partners.
• Clinical presentation
 Primary CMV infection usually is asymptomatic or mild URTI in immunocompetent hosts, after which the virus becomes latent. CMV community-acquired pneumonia, however, has been reported in immunocompetent hosts and may be severe.32
Primary CMV infection usually is asymptomatic or mild URTI in immunocompetent hosts, after which the virus becomes latent. CMV community-acquired pneumonia, however, has been reported in immunocompetent hosts and may be severe.32
 Severe disease tends to occur in immunocompromised patients. Solid organ and bone marrow transplantation patients are at highest risk of infection during the first 100 days after transplantation.
Severe disease tends to occur in immunocompromised patients. Solid organ and bone marrow transplantation patients are at highest risk of infection during the first 100 days after transplantation.
 CMV can also involve the liver, spleen, GI tract, central nervous system, or bone marrow.
CMV can also involve the liver, spleen, GI tract, central nervous system, or bone marrow.
 CMV has been shown in multiple studies to infect alveolar epithelial cells, and the main pulmonary manifestation is CMV pneumonitis, which may occur in up to 15% of transplant populations. The disease is usually severe with hypoxemia, a high incidence of respiratory failure and a mortality rate exceeding 80%. Patients may present with focal infiltrates, bilateral patchy infiltrates, or diffuse interstitial infiltrates.
CMV has been shown in multiple studies to infect alveolar epithelial cells, and the main pulmonary manifestation is CMV pneumonitis, which may occur in up to 15% of transplant populations. The disease is usually severe with hypoxemia, a high incidence of respiratory failure and a mortality rate exceeding 80%. Patients may present with focal infiltrates, bilateral patchy infiltrates, or diffuse interstitial infiltrates.
 CMV may reactivate in the chronically ventilated ICU patient.
CMV may reactivate in the chronically ventilated ICU patient.
 Pulmonary manifestations are also seen in HIV patients but have become less prevalent with the advent of highly active antiretroviral therapy. CMV is often identified in patients presenting with Pneumocystis jiroveci pneumonia, although the virus may not contribute to the severity of disease or symptoms.
Pulmonary manifestations are also seen in HIV patients but have become less prevalent with the advent of highly active antiretroviral therapy. CMV is often identified in patients presenting with Pneumocystis jiroveci pneumonia, although the virus may not contribute to the severity of disease or symptoms.
 Clinical laboratory features of CMV include elevation of serum transaminases, relative lymphopenia, atypical lymphocytes, and thrombocytopenia and suggest CMV over other viral infections.
Clinical laboratory features of CMV include elevation of serum transaminases, relative lymphopenia, atypical lymphocytes, and thrombocytopenia and suggest CMV over other viral infections.
• Diagnosis
 PCR has a high sensitivity and specificity for detecting viremia and is useful for monitoring resolution, although serology demonstrating an elevated CMV IgM level or fourfold increase in IgG levels is still the most common diagnostic test.
PCR has a high sensitivity and specificity for detecting viremia and is useful for monitoring resolution, although serology demonstrating an elevated CMV IgM level or fourfold increase in IgG levels is still the most common diagnostic test.
 Isolation of CMV from sputum culture, bronchial washings, and BAL fluid is diagnostic, but CMV grows very slowly and confirmation can take several days and up to 3 weeks.
Isolation of CMV from sputum culture, bronchial washings, and BAL fluid is diagnostic, but CMV grows very slowly and confirmation can take several days and up to 3 weeks.
 CMV pneumonitis is confirmed histologically (obtained by bronchoscopy or surgery) as giant CMV-infected pneumocyte inclusions. Immunohistochemical stains for CMV have also been developed.
CMV pneumonitis is confirmed histologically (obtained by bronchoscopy or surgery) as giant CMV-infected pneumocyte inclusions. Immunohistochemical stains for CMV have also been developed.
• Treatment33
 First line: ganciclovir (5 mg/kg IV q12h).
First line: ganciclovir (5 mg/kg IV q12h).
 Oral valganciclovir (900 mg PO q12h) can also be used in some cases, or as an extension following induction therapy with IV ganciclovir. Therapy typically lasts 21 days.
Oral valganciclovir (900 mg PO q12h) can also be used in some cases, or as an extension following induction therapy with IV ganciclovir. Therapy typically lasts 21 days.
 Other agents effective against CMV include foscarnet, cidofovir, and CMV immune globulin (cytogam).
Other agents effective against CMV include foscarnet, cidofovir, and CMV immune globulin (cytogam).
 Foscarnet is nephrotoxic and usually reserved for cases of ganciclovir-resistant CMV.
Foscarnet is nephrotoxic and usually reserved for cases of ganciclovir-resistant CMV.
 Cytogam is reserved for cases of life-threatening CMV infection (e.g., transplant patients with severe disease). See Chapter 29, for more information on diagnosis and treatment of CMV infection in this patient population.
Cytogam is reserved for cases of life-threatening CMV infection (e.g., transplant patients with severe disease). See Chapter 29, for more information on diagnosis and treatment of CMV infection in this patient population.
Herpesviruses: HSV-1, EBV, VZV
• The Herpesviridae family includes HSV-1, HSV-2, VZV, EBV, and human herpesvirus-8 (HHV-8, the causative agent of Kaposi sarcoma).
• In certain ICU populations (immunocompromised, ARDS, chronically ventilated, postsurgical, or burn), HSV-1 is an important cause of upper (and possibly lower) respiratory tract infection.
• EBV infection has been implicated in the development of posttransplant lymphoproliferative disease (see Chapter 29).
• VZV manifests with chickenpox on primary exposure and as zoster with reactivation. Primary pneumonias are rare but have a high mortality rate. Immunocompromised patients are at greatest risk of VZV pulmonary infection. Infections are treated with IV acyclovir. Preventative measures in immunocompromised patients may include vaccination of seronegative patients before transplantation, administration of varicella zoster immune globulin to exposed patients, or the use of prophylactic acyclovir.
Influenza Virus
• Virology
 Influenza is an acute respiratory illness caused by type A or type B influenza virus infection.
Influenza is an acute respiratory illness caused by type A or type B influenza virus infection.
 They are negative-strand, segmented RNA virus in the Orthomyxoviridae family, subdivided into antigenic subgroups based on the properties of the HA and NA glycoproteins.
They are negative-strand, segmented RNA virus in the Orthomyxoviridae family, subdivided into antigenic subgroups based on the properties of the HA and NA glycoproteins.
 Influenza viruses circulate in humans, birds, and swine. New influenza strains can arise from reassortment of viral gene products in coinfected organisms (e.g., a pig coinfected with an avian strain and a swine strain).
Influenza viruses circulate in humans, birds, and swine. New influenza strains can arise from reassortment of viral gene products in coinfected organisms (e.g., a pig coinfected with an avian strain and a swine strain).
• Epidemiology
 Influenza traditionally occurs in a seasonal, epidemic form in the winter months.
Influenza traditionally occurs in a seasonal, epidemic form in the winter months.
 Pandemic influenza arises periodically with strains more highly pathogenic than the seasonal variants (e.g., 1918 H1N1 Spanish influenza pandemic that resulted in 20–40 million deaths worldwide, 1957 H2N2 Asian influenza, 1968 H3N2 Hong Kong influenza, and 2009 H1N1 influenza).34–36
Pandemic influenza arises periodically with strains more highly pathogenic than the seasonal variants (e.g., 1918 H1N1 Spanish influenza pandemic that resulted in 20–40 million deaths worldwide, 1957 H2N2 Asian influenza, 1968 H3N2 Hong Kong influenza, and 2009 H1N1 influenza).34–36
• Clinical presentation
 Most cases of influenza are mild, self-limited upper respiratory infections. The incubation period ranges from 1 to 3 days.
Most cases of influenza are mild, self-limited upper respiratory infections. The incubation period ranges from 1 to 3 days.
 Typical symptoms include fever, myalgias, fatigue, and headache, with respiratory symptoms such as rhinorrhea, sore throat, and cough. GI symptoms, including vomiting, and diarrhea, may also occur, especially in children.
Typical symptoms include fever, myalgias, fatigue, and headache, with respiratory symptoms such as rhinorrhea, sore throat, and cough. GI symptoms, including vomiting, and diarrhea, may also occur, especially in children.
 Severe cases may present with dyspnea, hemoptysis, purulent sputum, rapidly progressive hypoxemia, primary and secondary pneumonia, respiratory failure, ARDS, multiorgan failure, and even death.
Severe cases may present with dyspnea, hemoptysis, purulent sputum, rapidly progressive hypoxemia, primary and secondary pneumonia, respiratory failure, ARDS, multiorgan failure, and even death.
 Pneumonia may be primary (viral pneumonitis) or secondary to bacterial coinfection, notably Staphylococcus aureus, S. pneumoniae, S. pyogenes, and H. influenzae. Patients with primary influenza pneumonia usually have persistent or worsening symptoms, very high fevers, and dyspnea with or without cyanosis, whereas patients with secondary bacterial pneumonias may show some improvement in their influenza-related symptoms before developing worsening fever and respiratory complaints.
Pneumonia may be primary (viral pneumonitis) or secondary to bacterial coinfection, notably Staphylococcus aureus, S. pneumoniae, S. pyogenes, and H. influenzae. Patients with primary influenza pneumonia usually have persistent or worsening symptoms, very high fevers, and dyspnea with or without cyanosis, whereas patients with secondary bacterial pneumonias may show some improvement in their influenza-related symptoms before developing worsening fever and respiratory complaints.
 Postinfluenza asthenia refers to weakness and fatigue after an influenza infection that may last several weeks.
Postinfluenza asthenia refers to weakness and fatigue after an influenza infection that may last several weeks.
• Diagnosis
 Clinical symptoms during seasonal epidemics provide a high degree of clinical suspicion.
Clinical symptoms during seasonal epidemics provide a high degree of clinical suspicion.
 Common radiographic findings include bilateral patchy, interstitial, and alveolar infiltrates, predominantly in the lower lobes. Ground-glass opacities may also be present.
Common radiographic findings include bilateral patchy, interstitial, and alveolar infiltrates, predominantly in the lower lobes. Ground-glass opacities may also be present.
 Rapid detection of influenza can be made by RT-PCR. Multiple testing of samples from various sites (nasopharyngeal swab, tracheal aspirate, and bronchial lavage) can improve diagnostic yield.
Rapid detection of influenza can be made by RT-PCR. Multiple testing of samples from various sites (nasopharyngeal swab, tracheal aspirate, and bronchial lavage) can improve diagnostic yield.
 Viral culture may take up to a week for positive identification and is thus not as useful.
Viral culture may take up to a week for positive identification and is thus not as useful.
• Treatment
 Treatment is largely supportive.
Treatment is largely supportive.
 Acetaminophen is recommended over salicylates (to avoid Reye syndrome in patients <18 years old.) Antitussives may also be used, and adequate hydration is essential.
Acetaminophen is recommended over salicylates (to avoid Reye syndrome in patients <18 years old.) Antitussives may also be used, and adequate hydration is essential.
 Antibiotics are reserved for bacterial superinfections, including pneumonia, otitis media, and sinusitis.
Antibiotics are reserved for bacterial superinfections, including pneumonia, otitis media, and sinusitis.
 NA inhibitors are most effective if prescribed with 48 hours of symptom onset.
NA inhibitors are most effective if prescribed with 48 hours of symptom onset.
 Oseltamivir (75 mg bid × 5 days) has activity against both type A and type B influenza viruses and can reduce viral shedding, symptom duration, length of hospitalization, and even mortality in severe cases. Treatment is generally 5 days in length. Higher doses (150 mg bid) and longer durations can be used in more severe disease.37–39
Oseltamivir (75 mg bid × 5 days) has activity against both type A and type B influenza viruses and can reduce viral shedding, symptom duration, length of hospitalization, and even mortality in severe cases. Treatment is generally 5 days in length. Higher doses (150 mg bid) and longer durations can be used in more severe disease.37–39
 Zanamivir (10 mg inhaled bid for 5 days) can be used to treat influenza in patients over 7 years old. It can cause bronchospasm and should be avoided in patients with chronic pulmonary disease.39
Zanamivir (10 mg inhaled bid for 5 days) can be used to treat influenza in patients over 7 years old. It can cause bronchospasm and should be avoided in patients with chronic pulmonary disease.39
 Amantadine (100 mg PO bid for 5 days) and rimantadine (100 mg PO bid for 5 days) are active only against influenza A, are less effective (75–100%), and recommended as second-line agents. These medications act against the viral M2 protein.
Amantadine (100 mg PO bid for 5 days) and rimantadine (100 mg PO bid for 5 days) are active only against influenza A, are less effective (75–100%), and recommended as second-line agents. These medications act against the viral M2 protein.
• Prevention
 Immunization is the most effective prevention for influenza. Options include injectable inactivated split-virus injected (recommended for all people >6 months old) and live attenuated virus inhaled (for healthy, nonpregnant people aged 2–49). Live vaccines should be avoided in patients who are immunosuppressed. As previously noted, flublok is a recently approved egg-free, trivalent, recombinant HA, injectable influenza vaccine.
Immunization is the most effective prevention for influenza. Options include injectable inactivated split-virus injected (recommended for all people >6 months old) and live attenuated virus inhaled (for healthy, nonpregnant people aged 2–49). Live vaccines should be avoided in patients who are immunosuppressed. As previously noted, flublok is a recently approved egg-free, trivalent, recombinant HA, injectable influenza vaccine.
 Flu vaccines are recommended, especially in pregnant women, children <5 years old, adults >50 years old, people with chronic medical conditions (e.g., chronic respiratory disease, cardiovascular disease, diabetes, obesity, renal disease, immunosuppression), healthcare workers, residents of long-term care facilities, and people who live with or care for patients at high risk for severe influenza (including children under 6 months of age who cannot be vaccinated).40
Flu vaccines are recommended, especially in pregnant women, children <5 years old, adults >50 years old, people with chronic medical conditions (e.g., chronic respiratory disease, cardiovascular disease, diabetes, obesity, renal disease, immunosuppression), healthcare workers, residents of long-term care facilities, and people who live with or care for patients at high risk for severe influenza (including children under 6 months of age who cannot be vaccinated).40
 Flu vaccines are not recommended for patients with serious documented allergies to eggs or egg components (excepting flublok), previous reaction to an influenza vaccine, children younger than 6 months old, and patients who are acutely ill.
Flu vaccines are not recommended for patients with serious documented allergies to eggs or egg components (excepting flublok), previous reaction to an influenza vaccine, children younger than 6 months old, and patients who are acutely ill.
 Chemoprophylaxis can be used with both oseltamivir (in patients ≥1 year old) and zanamivir (in patients >5 years old). Dosing is once daily for 7 days. In long-term care facilities and hospitals, chemoprophylaxis should be provided for a minimum of 2 weeks and up to 1 week after the date of the last diagnosed case.41
Chemoprophylaxis can be used with both oseltamivir (in patients ≥1 year old) and zanamivir (in patients >5 years old). Dosing is once daily for 7 days. In long-term care facilities and hospitals, chemoprophylaxis should be provided for a minimum of 2 weeks and up to 1 week after the date of the last diagnosed case.41
Metapneumovirus
• Virology: hMPV is a member of the Paramyxoviridae (like RSV and PIV).
• Epidemiology: It was discovered in 2001 and is now recognized as a cause of upper and lower respiratory tract infections, primarily in children of whom 90–100% have evidence of infection by age 10. hMPV has been implicated in 10% of all hospitalized cases of respiratory viral infections.
• Clinical presentation ranges from the cold-like symptoms to bronchiolitis and severe pneumonia. Risk factors for severe disease include immunosuppression, elderly, and prematurity and underlying cardiopulmonary disease in children. hMPV can also trigger exacerbations of asthma and other chronic cardiopulmonary diseases.
• Diagnosis is by RT-PCR, direct immunofluorescence, and/or viral culture.
• Treatment: There are no approved targeted therapies, although studies of ribavirin and neutralizing monoclonal antibodies are in progress.42
Nipah Virus
• Nipah virus (NiV), like hMPV, is a newly emergent member of the Paramyxoviridae family and Henipavirus genus. Hendra virus and Cedar virus are also a member of this genus.
• The original outbreak of NiV in Malaysia and Singapore in 1999 had a case fatality rate of nearly 40%. More recent outbreaks in Bangladesh and India have been more severe with case fatality rates reaching 90%. There is concern NiV could be spread due to the global nature of today’s transportation systems.43,44
• Transmission is likely through infected secretions, foodborne and fomite transmissions.
• Clinical presentation is rapidly progressive encephalitis and/or severe respiratory illness.
• Currently there is no treatment or vaccine for NiV. Reports from the original outbreaks suggested that patients treated with ribavirin had lower mortality but further study is warranted.
Parainfluenza Virus
• Virology: PIV is another member of Paramyxoviridae with four major serotypes: PIV1, PIV2, PIV3, and PIV4. PIV3 is the most prevalent serotype, with 90–100% of children being seropositive by age 5.
• Epidemiology
 PIV causes ∼20% of acute respiratory tract infections in hospitalized children, ranking as the second most common etiology for lower respiratory tract infections in this patient population. Up to 10% of acute lower respiratory tract infections in hospitalized adults can be attributed to PIV.
PIV causes ∼20% of acute respiratory tract infections in hospitalized children, ranking as the second most common etiology for lower respiratory tract infections in this patient population. Up to 10% of acute lower respiratory tract infections in hospitalized adults can be attributed to PIV.
 Spread of the virus occurs through large droplet inhalation, and the virus is easily spread by person-to-person contact.
Spread of the virus occurs through large droplet inhalation, and the virus is easily spread by person-to-person contact.
 PIV1 causes epidemics every 2 years during the fall, while PIV3 occurs in annual spring and summer epidemics. PIV2 and PIV4 occur in less predictable patterns. However, studies in adults suggest year-round circulation of all four serotypes.45
PIV1 causes epidemics every 2 years during the fall, while PIV3 occurs in annual spring and summer epidemics. PIV2 and PIV4 occur in less predictable patterns. However, studies in adults suggest year-round circulation of all four serotypes.45
• Clinical presentation
 PIV causes upper and lower respiratory infections in adults and children. Primarily infects nasal and oropharyngeal epithelial cells, with subsequent distal spread to the large and small airways. The incubation period is typically 2–8 days.
PIV causes upper and lower respiratory infections in adults and children. Primarily infects nasal and oropharyngeal epithelial cells, with subsequent distal spread to the large and small airways. The incubation period is typically 2–8 days.
 PIV1 and PIV2 are the primary causes of childhood croup (fever, rhinitis, pharyngitis, with a barking cough). Symptoms last up to 4 days. Stridor, dyspnea, and respiratory distress can develop in severe cases.
PIV1 and PIV2 are the primary causes of childhood croup (fever, rhinitis, pharyngitis, with a barking cough). Symptoms last up to 4 days. Stridor, dyspnea, and respiratory distress can develop in severe cases.
 PIV3 is associated with bronchiolitis and pneumonia and is often mistaken for RSV infection.
PIV3 is associated with bronchiolitis and pneumonia and is often mistaken for RSV infection.
 In adults, PIV usually causes a mild URTI but lower respiratory disease is possible, especially in the setting of chronic lung disease.
In adults, PIV usually causes a mild URTI but lower respiratory disease is possible, especially in the setting of chronic lung disease.
 PIV has been associated with COPD and asthma exacerbations.
PIV has been associated with COPD and asthma exacerbations.
 Immunocompromised hosts are susceptible to serious PIV infections, including pneumonia or even disseminated infections. Bone marrow, stem cell, and less commonly solid organ transplantation recipients may have PIV infections and a relatively high mortality.45–47
Immunocompromised hosts are susceptible to serious PIV infections, including pneumonia or even disseminated infections. Bone marrow, stem cell, and less commonly solid organ transplantation recipients may have PIV infections and a relatively high mortality.45–47
 Complications may include secondary bacterial pneumonia, sinusitis, otitis media, meningitis, pericarditis, myocarditis, and Guillain–Barré syndrome. The last three complications are very rare.
Complications may include secondary bacterial pneumonia, sinusitis, otitis media, meningitis, pericarditis, myocarditis, and Guillain–Barré syndrome. The last three complications are very rare.
• Diagnosis is often made clinically, although RT-PCR has become more common. As with other respiratory viruses, viral culture is the gold standard but clinically not useful.
• Treatment
 Treatment is largely supportive.
Treatment is largely supportive.
 There are no antiviral agents with specific activity against PIV. Ribavirin has been used to treat bone marrow transplant recipients but few data exist on its efficacy. Combination therapy with ribavirin and immunoglobulin has not altered the duration of illness or mortality in clinical studies.48
There are no antiviral agents with specific activity against PIV. Ribavirin has been used to treat bone marrow transplant recipients but few data exist on its efficacy. Combination therapy with ribavirin and immunoglobulin has not altered the duration of illness or mortality in clinical studies.48
 Steroids (dexamethasone 0.6 mg/kg PO or IV) recommended in children with severe croup can decrease length of hospitalization, need for additional therapies, and need for intubation. Nebulized epinephrine can be added if stridor or respiratory distress is present.49
Steroids (dexamethasone 0.6 mg/kg PO or IV) recommended in children with severe croup can decrease length of hospitalization, need for additional therapies, and need for intubation. Nebulized epinephrine can be added if stridor or respiratory distress is present.49
Respiratory Syncytial Virus
• Virology
 RSV is an enveloped, single-stranded, negative-sense RNA virus of the Paramyxoviridae family.
RSV is an enveloped, single-stranded, negative-sense RNA virus of the Paramyxoviridae family.
 RSV can be divided into two distinct antigenic groups (RSV-A and RSV-B), both of which are present during outbreaks.
RSV can be divided into two distinct antigenic groups (RSV-A and RSV-B), both of which are present during outbreaks.
 RSV primarily infects airway epithelial cells. Following cellular entry and replication, the virus is transmitted from cell to cell through fusion of neighboring cells into large multinucleated syncytia.
RSV primarily infects airway epithelial cells. Following cellular entry and replication, the virus is transmitted from cell to cell through fusion of neighboring cells into large multinucleated syncytia.
• Epidemiology
 RSV is the most common cause of LRIs in infants and young children. Highest rates of illness are seen in infants aged 1–6 months, with peak rates occurring at 3 months. Risk factors include preterm birth, male sex, immunodeficiency, lack of breastfeeding, and overcrowding.50
RSV is the most common cause of LRIs in infants and young children. Highest rates of illness are seen in infants aged 1–6 months, with peak rates occurring at 3 months. Risk factors include preterm birth, male sex, immunodeficiency, lack of breastfeeding, and overcrowding.50
 In adults, RSV is an underrecognized cause of LRI with >5% of adult LRIs attributable to RSV, especially elderly and immunocompromised patients.51
In adults, RSV is an underrecognized cause of LRI with >5% of adult LRIs attributable to RSV, especially elderly and immunocompromised patients.51
 Outbreaks occur in late fall through early spring.
Outbreaks occur in late fall through early spring.
 RSV transmission occurs by contact with respiratory secretions or large respiratory droplets and fomites.
RSV transmission occurs by contact with respiratory secretions or large respiratory droplets and fomites.
 Previous infection with RSV does not confer complete protection against reinfection. Humoral immunity, however, may reduce the severity of subsequent RSV infections. Elderly patients who have lower antibody titers are more likely to develop symptomatic disease.
Previous infection with RSV does not confer complete protection against reinfection. Humoral immunity, however, may reduce the severity of subsequent RSV infections. Elderly patients who have lower antibody titers are more likely to develop symptomatic disease.
• Clinical Presentation
 RSV URTIs present with cough, coryza, rhinorrhea, conjunctivitis, and otitis media.
RSV URTIs present with cough, coryza, rhinorrhea, conjunctivitis, and otitis media.
 Apneic episodes may be seen in infants admitted with RSV infections but the exact mechanism remains unclear and may precipitate sudden infant death syndrome. Prevalence estimates for apnea vary widely.20
Apneic episodes may be seen in infants admitted with RSV infections but the exact mechanism remains unclear and may precipitate sudden infant death syndrome. Prevalence estimates for apnea vary widely.20
 LRIs present with bronchospasm, bronchiolitis, pneumonia, and in severe cases, respiratory failure. Patients at risk for lower respiratory tract disease include infants (<6 months of age), children with underlying structural lung and heart disease, patients of any age group with significant asthma or COPD, institutionalized elderly patients, and immunocompromised patients.
LRIs present with bronchospasm, bronchiolitis, pneumonia, and in severe cases, respiratory failure. Patients at risk for lower respiratory tract disease include infants (<6 months of age), children with underlying structural lung and heart disease, patients of any age group with significant asthma or COPD, institutionalized elderly patients, and immunocompromised patients.
 RSV has been associated with the development of asthma in children.51
RSV has been associated with the development of asthma in children.51
• Diagnosis
 RT-PCR is the diagnostic test of choice because of its rapidity, sensitivity, and specificity.
RT-PCR is the diagnostic test of choice because of its rapidity, sensitivity, and specificity.
 Viral culture from nasal or throat swabs, tracheal aspirates, bronchial washings, or BAL specimens remains the gold standard for diagnosis. Culture may take days to weeks before identification of virus by immunofluorescence staining.
Viral culture from nasal or throat swabs, tracheal aspirates, bronchial washings, or BAL specimens remains the gold standard for diagnosis. Culture may take days to weeks before identification of virus by immunofluorescence staining.
 Diagnostic serology is unhelpful because individuals may have high levels of antibodies in circulation from previous RSV infections.
Diagnostic serology is unhelpful because individuals may have high levels of antibodies in circulation from previous RSV infections.
• Treatment
 Treatment is supportive with oxygen supplementation and fluid resuscitation.20
Treatment is supportive with oxygen supplementation and fluid resuscitation.20
 Bronchodilators may be used in adults with underlying asthma/COPD. Nebulized epinephrine may improve airway obstruction in RSV bronchiolitis. Neither is recommended for infants and children with bronchiolitis.20
Bronchodilators may be used in adults with underlying asthma/COPD. Nebulized epinephrine may improve airway obstruction in RSV bronchiolitis. Neither is recommended for infants and children with bronchiolitis.20
 Mechanical ventilation may be required in some patient with severe disease.
Mechanical ventilation may be required in some patient with severe disease.
 The evidence for steroids is not compelling but may still be used in cases of significant wheezing.20,23
The evidence for steroids is not compelling but may still be used in cases of significant wheezing.20,23
 Ribavirin, a synthetic nucleoside analog, may be considered in:
Ribavirin, a synthetic nucleoside analog, may be considered in:
 Infants with structural heart or lung disease, immunosuppressed, or with mechanical ventilation.
Infants with structural heart or lung disease, immunosuppressed, or with mechanical ventilation.
 In bone marrow and stem cell transplantation recipients, the early use of ribavirin has been shown to reduce morbidity and mortality compared to historic controls.52–54
In bone marrow and stem cell transplantation recipients, the early use of ribavirin has been shown to reduce morbidity and mortality compared to historic controls.52–54
 Ribavirin has no proven efficacy in immunocompetent adults but use may be considered in severe cases of respiratory failure.
Ribavirin has no proven efficacy in immunocompetent adults but use may be considered in severe cases of respiratory failure.
 Ribavirin is contraindicated in pregnancy.
Ribavirin is contraindicated in pregnancy.
 Palivizumab, an RSV-specific humanized monoclonal antibody, is primarily used for infants.
Palivizumab, an RSV-specific humanized monoclonal antibody, is primarily used for infants.
 In immunocompromised adults, studies of combined therapy of palivizumab and ribavirin suggest improved outcome.55–57
In immunocompromised adults, studies of combined therapy of palivizumab and ribavirin suggest improved outcome.55–57
 Motavizumab, a second-generation RSV-specific monoclonal antibody, is currently in trials.58
Motavizumab, a second-generation RSV-specific monoclonal antibody, is currently in trials.58
 Preventive palivizumab is recommended during the first year of life for infants with hemodynamically significant heart disease or chronic lung disease of prematurity.20
Preventive palivizumab is recommended during the first year of life for infants with hemodynamically significant heart disease or chronic lung disease of prematurity.20
 Other therapies currently in development include RSV fusion protein inhibitors, RSV polymerase inhibitors, small interfering RNA (siRNA), leukotriene antagonists, and surfactant.
Other therapies currently in development include RSV fusion protein inhibitors, RSV polymerase inhibitors, small interfering RNA (siRNA), leukotriene antagonists, and surfactant.
 RSV IV immunoglobulin (IVIG) is probably not helpful and is no longer available.59
RSV IV immunoglobulin (IVIG) is probably not helpful and is no longer available.59
Rhinovirus
• Virology
 Rhinoviruses are nonenveloped, single-stranded RNA viruses in the Picornaviridae family.
Rhinoviruses are nonenveloped, single-stranded RNA viruses in the Picornaviridae family.
 They preferentially grow at 33–35°C, the temperature in the nasal passages and upper airways.
They preferentially grow at 33–35°C, the temperature in the nasal passages and upper airways.
 There are 100 different serotypes in RV-A and RV-B. A third lineage, RV-C, discovered in 2006 includes over 50 strains. No one strain predominantly circulates at any one time.
There are 100 different serotypes in RV-A and RV-B. A third lineage, RV-C, discovered in 2006 includes over 50 strains. No one strain predominantly circulates at any one time.
• Epidemiology
 Rhinoviruses are responsible for about half of common colds.
Rhinoviruses are responsible for about half of common colds.
 Infection rates are highest among young children and infants, with an average of six infections per year. Infection rates decrease with advancing age except in the 20s, when another peak is seen. Risks for infections include contact with small children aged <6 years.
Infection rates are highest among young children and infants, with an average of six infections per year. Infection rates decrease with advancing age except in the 20s, when another peak is seen. Risks for infections include contact with small children aged <6 years.
 Rhinovirus infections peak in autumn and spring in North America and Europe. They are more active during the rainy season in tropical regions of the world.
Rhinovirus infections peak in autumn and spring in North America and Europe. They are more active during the rainy season in tropical regions of the world.
 Transmission is via direct contact with infected secretions. Subsequent self-inoculation of the nasal or conjunctival mucosa leads to infection. Transmission by fomites and aerosolized virus may also occur.
Transmission is via direct contact with infected secretions. Subsequent self-inoculation of the nasal or conjunctival mucosa leads to infection. Transmission by fomites and aerosolized virus may also occur.
 Despite any anecdotal evidence and popular beliefs, cold temperature exposure, fatigue, and sleep deprivation have not been associated with increased rates of transmission.
Despite any anecdotal evidence and popular beliefs, cold temperature exposure, fatigue, and sleep deprivation have not been associated with increased rates of transmission.
 Diligent hand washing can effectively prevent the spread of rhinoviruses.
Diligent hand washing can effectively prevent the spread of rhinoviruses.
• Clinical presentation
 Incubation period is 1–2 days.
Incubation period is 1–2 days.
 Symptoms may include rhinorrhea and sneezing (50–70%), sore throat (50%), malaise, mild headaches, and otitis media in some cases. Symptoms generally last about a week to 10 days but minor symptomatology can linger longer and viral shedding may occur up to 3 weeks following infection.60
Symptoms may include rhinorrhea and sneezing (50–70%), sore throat (50%), malaise, mild headaches, and otitis media in some cases. Symptoms generally last about a week to 10 days but minor symptomatology can linger longer and viral shedding may occur up to 3 weeks following infection.60
 Hoarseness and cough are less common but can occur due to upper airway irritation or concurrently with sinusitis and bronchitis. Fever, chills, and myalgias are unusual and should prompt the clinician to search for other potential causes, such as influenza.
Hoarseness and cough are less common but can occur due to upper airway irritation or concurrently with sinusitis and bronchitis. Fever, chills, and myalgias are unusual and should prompt the clinician to search for other potential causes, such as influenza.
 The nasal mucosa becomes edematous and hyperemic and produces a mucoid discharge. The nasal turbinates often become engorged, which can lead to sinus cavity obstruction and occasionally bacterial superinfection.
The nasal mucosa becomes edematous and hyperemic and produces a mucoid discharge. The nasal turbinates often become engorged, which can lead to sinus cavity obstruction and occasionally bacterial superinfection.
 Rhinovirus may also infect the lower respiratory tract, although causation is hard to discern as coinfection with other viruses is fairly common.
Rhinovirus may also infect the lower respiratory tract, although causation is hard to discern as coinfection with other viruses is fairly common.
 Rhinovirus infections are associated with exacerbations of asthma, COPD, and cystic fibrosis.60
Rhinovirus infections are associated with exacerbations of asthma, COPD, and cystic fibrosis.60
• Diagnosis: Rhinovirus infection is usually diagnosed clinically as a common cold, although with poor specificity. Viral diagnosis can be confirmed by RT-PCR from nasal swab or nasal washing but generally unnecessary.
• Treatment is supportive and symptom based.
Complications
• Postviral cough
 Cough is a common symptom during acute viral upper and lower respiratory tract infection.
Cough is a common symptom during acute viral upper and lower respiratory tract infection.
 Many cases of subacute cough (persisting for 3–8 weeks) are due to postviral cough, which may not respond well to common antitussive therapy.61
Many cases of subacute cough (persisting for 3–8 weeks) are due to postviral cough, which may not respond well to common antitussive therapy.61
 Although postviral cough is the most common etiology for subacute cough, other potential causes including postnasal drip, gastroesophageal reflux disease, eosinophilic airway inflammation, angiotensin-converting enzyme (ACE) inhibitor, and Bordetella pertussis should be evaluated and ruled out, particularly if the cough persists beyond 8 weeks.
Although postviral cough is the most common etiology for subacute cough, other potential causes including postnasal drip, gastroesophageal reflux disease, eosinophilic airway inflammation, angiotensin-converting enzyme (ACE) inhibitor, and Bordetella pertussis should be evaluated and ruled out, particularly if the cough persists beyond 8 weeks.
 In patients with asthma or COPD, cough may be a manifestation of prolonged exacerbation. In these patients, spirometry can guide therapy.
In patients with asthma or COPD, cough may be a manifestation of prolonged exacerbation. In these patients, spirometry can guide therapy.
• Postviral wheeze/asthma
 Postviral wheezing/asthma is more common in children and may continue up to 1 year after resolution of the infection.
Postviral wheezing/asthma is more common in children and may continue up to 1 year after resolution of the infection.
 For most patients wheezing will spontaneously resolve over time.
For most patients wheezing will spontaneously resolve over time.
 Multiple studies have implicated infantile rhinovirus and RSV infections in the subsequent development of asthma in children and teens.62
Multiple studies have implicated infantile rhinovirus and RSV infections in the subsequent development of asthma in children and teens.62
REFERENCES
1. Gwaltney JM Jr. Acute community-acquired sinusitis. Clin Infect Dis. 1996;23:1209–23.
2. Rosenfeld RM, Andes D, Bhattacharyya N, et al. Clinical practice guideline: adult sinusitis. Otolaryngol Head Neck Surg. 2007;137:S1–31.
3. Meltzer EO, Hamilos DL. Rhinosinusitis diagnosis and management for the clinician: a synopsis of recent consensus guidelines. Mayo Clin Proc. 2011;86:427–43.
4. Chow AW, Benninger MS, Brook I, et al. IDSA clinical practice guideline for acute bacterial rhinosinusitis in children and adults. Clin Infect Dis. 2012;54:e72–112.
5. Gwaltney JM Jr, Phillips D, Miller D, et al. Computed tomographic study of the common cold. N Engl J Med. 1994;330:25–30.
6. Zalmanovici Trestioreanu A, Yaphe J. Intranasal steroids for acute sinusitis. Cochrane Database Syst Rev. 2013;12:CD005149.
7. Shulman ST, Bisno AL, Clegg HW, et al. Clinical practice guideline for the diagnosis and management of group A streptococcal pharyngitis: 2012 update by the Infectious Diseases Society of America. Clin Infect Dis. 2012;55:1279–82.
8. Centor RM, Witherspoon JM, Dalton HP, et al. The diagnosis of strep throat in adults in the emergency room. Med Decis Making. 1981;1:239–46.
9. McIsaac WJ, White D, Tannenbaum D, et al. A clinical score to reduce unnecessary antibiotic use in patients with sore throat. CMAJ. 1998;158:75–83.
10. McIsaac WJ, Goel V, To T, et al. The validity of a sore throat score in family practice. CMAJ. 2000;163:811–5.
11. McIsaac WJ, Kellner JD, Aufricht P, et al. Empirical validation of guidelines for the management of pharyngitis in children and adults. JAMA. 2004;291:1587–95.
12. Tiwari T, Murphy TV, Moran J. Recommended antimicrobial agents for the treatment and postexposure prophylaxis of pertussis: 2005 CDC guidelines. MMWR Recomm Rep. 2005;54 (RR-14):1–16.
13. Wenzel RP, Fowler AA 3rd. Clinical practice. Acute bronchitis. N Engl J Med. 2006;355:2125–30.
14. Snow V, Mottur-Pilson C, Gonzales R. Principles of appropriate antibiotic use for treatment of acute bronchitis in adults. Ann Intern Med. 2001;134:518–20.
15. Gonzales R, Bartlett JG, Besser RE, et al. Principles of appropriate antibiotic use for treatment of uncomplicated acute bronchitis: background. Ann Intern Med. 2001;134:521–9.
16. Braman SS. Chronic cough due to acute bronchitis: ACCP evidence-based clinical practice guidelines. Chest. 2006;129:95S–103S.
17. Smith SM, Fahey T, Smucny J, et al. Antibiotics for acute bronchitis. Cochrane Database Syst Rev. 2014;3:CD000245.
18. Becker LA, Hom J, Villasis-Keever M, et al. Beta2-agonists for acute cough or a clinical diagnosis of acute bronchitis. Cochrane Database Syst Rev. 2015;9:CD001726.
19. Schuh S. Update on management of bronchiolitis. Curr Opin Pediatr. 2011;23:110–4.
20. Ralston SL, Lieberthal AS, Meissner HC, et al. Clinical practice guideline: the diagnosis, management, and prevention of bronchiolitis. Pediatrics. 2014;134:e1474–502.
21. Donlan M, Fontela PS, Puligandla PS. Use of continuous positive airway pressure (CPAP) in acute viral bronchiolitis: a systematic review. Pediatr Pulmonol. 2011;46:736–46.
22. Wing R, James C, Maranda LS, et al. Use of high-flow nasal cannula support in the emergency department reduces the need for intubation in pediatric acute respiratory insufficiency. Pediatr Emerg Care. 2012;28:1117–23.
23. Fernandes RM, Bialy LM, Vandermeer B, et al. Glucocorticoids for acute viral bronchiolitis in infants and young children. Cochrane Database Syst Rev. 2010;CD004878.
24. Ruuskanen O, Lahti E, Jennings LC, et al. Viral pneumonia. The Lancet. 2011;377:1264–75.
25. Mandell LA, Wunderink RG, Anzueto A, et al. Infectious Diseases Society of America/American Thoracic Society consensus guidelines on the management of community-acquired pneumonia in adults. Clin Infect Dis. 2007;44:S27–72.
26. Longman RE, Johnson TRB. Viral respiratory disease in pregnancy. Curr Opin Obstet Gynecol. 2007;19:120–5.
27. DiazGrenados CA, Dunning AJ, Kimmel M, et al. Efficacy of high-dose versus standard-dose influenza vaccine in older adults. N Engl J Med. 2014;371:635–45.
28. Lynch JP III, Fishbein M, Echavarria M. Adenovirus. Semin Resp Crit Care Med. 2011;32:494–511.
29. Centers for Disease Control and Prevention (CDC). Revised U.S. surveillance case definition for severe acute respiratory syndrome (SARS) and update on SARS cases—United States and worldwide, December 2003. MMWR Morb Mortal Wkly Rep. 2003;52:1202–6.
30. Centers for Disease Control and Prevention (CDC). Severe acute respiratory syndrome (SARS). Available at http://www.cdc.gov/sars/. Accessed 11/4/15.
31. Wong SSY, Yuen KY. The management of coronavirus infections with particular reference to SARS. J Antimicrob Chemother. 2008;62:437–41.
32. Cunha BA. Cytomegalovirus pneumonia: community-acquired pneumonia in immunocompetent hosts. Infect Dis Clin N Am. 2010;24:147–58.
33. Kotton CN, Kumar D, Caliendo AM, et al. Updated international consensus guidelines on the management of cytomegalovirus in solid-organ transplantation. Transplantation. 2013;96:333–60.
34. Centers for Disease Control and Prevention. CDC estimates of 2009 H1N1 influenza cases, hospitalizations and deaths in the United States, April 2009–March 13, 2010. Available at http://www.cdc.gov/h1n1flu/estimates/April_March_13.htm. Accessed 11/4/15.
35. Bautista E, Chotpitayasunondh T, Gao Z, et al. Clinical aspects of pandemic 2009 influenza A (H1N1) virus infection. N Engl J Med. 2010;362:1708–19.
36. Ramsey C, Kumar A. H1N1: viral pneumonia as a cause of acute respiratory distress syndrome. Curr Opin Crit Care. 2011;17:64–71.
37. Dobson J, Whitley RJ, Pocock S, et al. Oseltamivir treatment for influenza in adults: a meta-analysis of randomised controlled trials. Lancet. 2015;385:1729–37.
38. Kumar A. Early versus late oseltamivir treatment in severely ill patients with 2009 pandemic influenza A (H1N1): speed is life. J Antimicrob Chemother. 2011;66:959–63.
39. Heneghan CJ, Onakpoya I, Thompson M, et al. Zanamivir for influenza in adults and children: systematic review of clinical study reports and summary of regulatory comments. BMJ. 2014; 348:g2547.
40. Centers for Disease Control and Prevention. Influenza (flu). Preventing seasonal flu with vaccination. Available at http://www.cdc.gov/flu/protect/vaccine/index.htm. Accessed 11/4/15.
41. Fiore AE, Fry A, Shay D, et al. Antiviral agents for the treatment and chemoprophylaxis of influenza—recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep. 2011;60:1–24.
42. Feuillet F, Lina B, Rosa-Calatrava M, et al. Ten years of human metapneumovirus research. J Clin Virol. 2012;53:97–105.
43. Lo MK, Rota PA. The emergence of Nipah virus, a highly pathogenic paramyxovirus. J Clin Virol. 2008;43:396–400.
44. Luby SP, Gurley ES. Epidemiology of henipavirus disease in humans. Curr Top Microbiol Immunol. 2012;359:25–40.
45. Hall CB. Respiratory syncytial virus and parainfluenza virus. N Engl J Med. 2001;344:1917–28.
46. Vilchez RA, Dauber J, McCurry K, et al. Parainfluenza virus infection in adult lung transplant recipients: an emergent clinical syndrome with implications on allograft function. Am J Transplant. 2003;3:116–20.
47. Chemaly RF, Hanmod SS, Rathod DB, et al. The characteristics and outcomes of parainfluenza virus infections in 200 patients with leukemia or recipients of hematopoietic stem cell transplantation. Blood. 2012;119:2738–45.
48. Nichols WG, Corey L, Gooley T, et al. Parainfluenza virus infections after hematopoietic stem cell transplantation: risk factors, response to antiviral therapy, and effect on transplant outcome. Blood. 2001;98:573–8.
49. Russell KF, Liang Y, O’Gorman K, et al. Glucocorticoids for croup. Cochrane Database Syst Rev. 2011:CD001955.
50. Sommer C, Resch B, Simões EA. Risk factors for severe respiratory syncytial virus lower respiratory tract infection. Open Microbiol J. 2011;5:144–54.
51. Falsey AR, Hennessey PA, Formica MA, et al. Respiratory syncytial virus infection in elderly and high-risk adults. N Engl J Med. 2005;352:1749–59.
52. McColl MD, Corser RB, Bremner J, et al. Respiratory syncytial virus infection in adult BMT recipients: effective therapy with short duration nebulised ribavirin. Bone Marrow Transplant. 1998;21:423–5.
53. Small TN, Casson A, Malak SF, et al. Respiratory syncytial virus infection following hematopoietic stem cell transplantation. Bone Marrow Transplant. 2002;29:321–7.
54. Waghmeare A, Campbell AP, Xie H, et al. Respiratory syncytial virus lower respiratory disease in hematopoietic cell transplant recipients: viral RNA detection in blood, antiviral treatment, and clinical outcomes. Clin Infect Dis. 2013;57:1731–41.
55. Boeckh M, Berrey MM, Bowden RA, et al. Phase 1 evaluation of the respiratory syncytial virus-specific monoclonal antibody palivizumab in recipients of hematopoietic stem cell transplants. J Infect Dis. 2011;184:350–4.
56. Walsh EE. Respiratory syncytial virus infection in adults. Semin Respir Crit Care Med. 2011;32:423–32.
57. Shah JN, Chemaly RF. Management of RSV infections in adult recipients of hematopoietic stem cell transplantation. Blood. 2011;117:2755–63.
58. Carbonell-Estrany X, Simões EA, Dagan R, et al. Motavizumab for prophylaxis of respiratory syncytial virus in high-risk children: a noninferiority trial. Pediatrics. 2010;125:e35–51.
59. Fuller H, Del Mar C. Immunoglobulin treatment for respiratory syncytial virus infection. Cochrane Database Syst Rev. 2006:CD004883.
60. Anzueto A, Niederman MS. Diagnosis and treatment of rhinovirus respiratory infections. Chest. 2003;123:1664–72.
61. Braman SS. Postinfectious cough: ACCP evidence-based clinical practice guidelines. Chest. 2006;129:138S–46S.
62. Feldman AS, He Y, Moore ML, et al. Toward primary prevention of asthma. Reviewing the evidence for early-life respiratory viral infections as modifiable risk factors to prevent childhood asthma. Am J Respir Crit Care Med. 2015;191:34–44.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


