Video-Assisted Thoracic Surgery for Diseases Within the Mediastinum
Alberto de Hoyos
Improved minimally invasive instrumentation and refinements in surgical techniques have allowed for increasing applications of video-assisted thoracic surgery (VATS) for the diagnostic evaluation and definitive surgical treatment of diseases of the mediastinum.7,94,115,176,177,178 Diagnostic procedures have been used principally for the evaluation of unknown masses of the anterior mediastinum, mediastinal lymphadenopathy, and confirmation of suspected hematopoietic malignancies such as lymphoma and Hodgkin’s disease. Therapeutic resections (usually without prior biopsy) have been primarily applied to patients with thymoma, myasthenia gravis, and neurogenic tumors as well as for the resection of a variety of mediastinal cysts. VATS techniques and the thoracic surgeon’s skills have advanced to the level that most mediastinal pathology can now be approached with VATS.
A detailed understanding of the anatomy and differential diagnosis of mediastinal pathology is essential for adequate management of these lesions. In this chapter, the role of VATS in the diagnosis and treatment of mediastinal diseases is reviewed, along with the indications and results of VATS procedures of the mediastinum (Table 171-1). VATS instrumentation, equipment, and basic techniques are reviewed separately in Chapter 33. More detailed description of specific mediastinal pathology is found in Chapters 162, 163, 164, 165, 166, 167, 168, 169 and 170 and 172, 173, 174, 175 and 176.
Video-Assisted Thoracic Surgical Management of Mediastinal Lymphadenopathy
The thoracic surgeon continues to maintain an important role in establishing the diagnosis of indeterminate mediastinal masses and lymphadenopathy. Improvements in the resolution of modern diagnostic radiographic modalities, specifically computed tomography (CT) and magnetic resonance imaging (MRI), have allowed for more accurate clinical (radiographic) evaluation of the mediastinum.46,56,122 Imaging modalities are, however, neither sufficiently sensitive nor specific in identifying lymph node metastasis prior to surgical exploration. The morphologic information obtained (i.e., lymph node size) does not differentiate between benign and malignant processes. By contrast, positron emission tomography (PET) with the metabolic tracer 18-fluorodeoxyglucose allows for functional characterization of tissues. Because malignant tissue, particularly non-small-cell lung cancer (NSCLC), is characterized by increased glucose metabolism, PET permits the visualization not only of the primary tumor but also of metastatic spread to regional lymph nodes. Measuring the amount of accumulated radioactivity in suspected lymph nodes using standardized uptake values (SUV) has helped improve the results further.36,69,96,223 Choosing a SUV of 2.5 as a threshold, false-positive and false-negative results are minimized. The sensitivity (96%) and specificity (81%) of PET compare favorably with those of CT for staging mediastinal lymph nodes (N0 + N1 versus N2 + N3).96,223 False-positive findings on PET (positive predictive value of 49.3%) may be seen in inflammatory conditions, and pathologic confirmation of positive findings is warranted before denying a patient potentially curative therapy. On the other hand, in view of the high negative predictive value of PET (98.4%), mediastinoscopy may be omitted in patients with small (<2 cm) and peripheral T1 lesions whose PET scan results are negative in the mediastinum. Pathologic evaluation of the mediastinal lymph nodes is indicated in the presence of N1 disease, T2 or T3 lesions, or in central T1 lesions with intense SUV uptake even in the presence of a negative mediastinal PET scan.39 False-negative PET studies may be seen in three specific settings: tumors with relatively low metabolic activity (bronchioalveolar carcinoma and carcinoid tumors), tumors <1.5 cm in size, and hyperglycemia. Integrated PET/CT provide enhanced accuracy and specificity in staging mediastinal lymph nodes as compared with CT/PET alone.20,195,221 Detterbeck and associates39 have summarized the guidelines for invasive staging of lung cancer. Traditional transbronchial needle biopsy and esophageal and endobronchial ultrasound-guided needle biopsy (EUS, EBUS) are becoming the preferred techniques to confirm cytologic metastatic involvement of mediastinal lymph nodes.43,44,70,71,91,145,227,230,241,242,243,244 These techniques are complementary and together can sample more mediastinal nodal stations than standard cervical mediastinoscopy; they are particularly useful in the assessment of posterior subcarinal, inferior mediastinal, and hilar lymphadenopathy, areas usually inaccessible by mediastinoscopy. However, a surgical biopsy is still required in many instances to establish a histologic diagnosis of mediastinal node involvement to rule out occult N2 disease (10%–16% of cases).4,135 Cervical mediastinoscopy
continues to be the preferred surgical diagnostic approach to most peritracheal and subcarinal mediastinal lymphadenopathy identified by preoperative CT scanning of the chest. However, as experience is gained with EUS and EBUS, these techniques may replace mediastinoscopy as the routine standard technique to sample mediastinal adenopathy. The technique of cervical mediastinoscopy is relatively simple, safe, and effective (Fig. 171-1). Accuracy and negative predictive value are improved by the routine addition of video capabilities to standard cervical mediastinoscopy.111
continues to be the preferred surgical diagnostic approach to most peritracheal and subcarinal mediastinal lymphadenopathy identified by preoperative CT scanning of the chest. However, as experience is gained with EUS and EBUS, these techniques may replace mediastinoscopy as the routine standard technique to sample mediastinal adenopathy. The technique of cervical mediastinoscopy is relatively simple, safe, and effective (Fig. 171-1). Accuracy and negative predictive value are improved by the routine addition of video capabilities to standard cervical mediastinoscopy.111
Table 171-1 Potential Indications of Mediastinal Pathology for Video-Assisted Thoracic Surgery (VATS) Intervention | ||
|---|---|---|
|
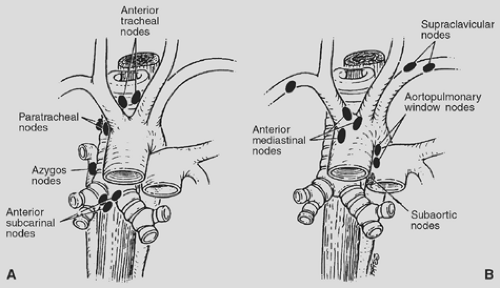 Figure 171-1. A: Standard lymph node stations accessible by standard cervical mediastinoscopy. B: Lymph node stations potentially accessible by extended cervical mediastinoscopy. |
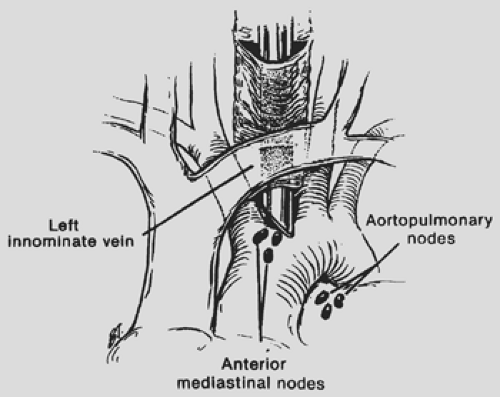
Limitations of the cervical mediastinoscopic approach for accessing the lymph nodes in the anterior mediastinum and aortopulmonary window stations led to parasternal or anterior mediastinotomy approaches.134,204 Familiar to most thoracic surgeons, the Chamberlain’s anterior mediastinotomy approach has become an effective adjunct to cervical mediastinoscopy in the evaluation of mediastinal lymphadenopathy. Although the introduction of anterior mediastinotomy was a welcome alternative to a standard thoracotomy to access the mediastinum, other investigators developed techniques of “extended” cervical mediastinoscopy to avoid the anterior mediastinotomy. Kirschner97 described the technique of passing the mediastinoscope anterior to the great vessels (Fig. 171-2), whereas Ginsberg and colleagues57 reported their method of passing the mediastinoscope between the innominate and the left carotid arteries to access the aortopulmonary window. Deslauriers and colleagues38 described a technique of “mediastino-pleuroscopy” (Fig. 171-3) to access the right pleural cavity and periazygos lymph nodes. However, the increased technical complexity and
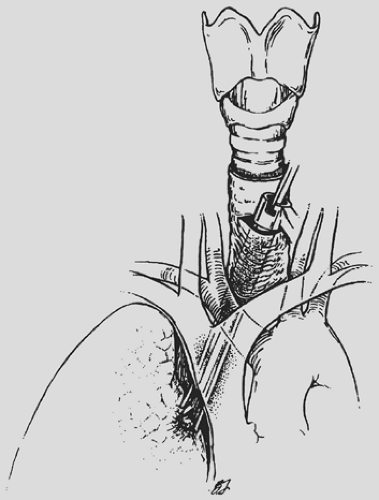
inability to assess low-lying nodes have limited widespread clinical application of the extended cervical mediastinoscopy techniques. For “bulky” mediastinal tumors in close proximity to the anterior chest wall, an anterior mediastinotomy (Chamberlain’s procedure) is the procedure of choice (Fig. 171-4).
inability to assess low-lying nodes have limited widespread clinical application of the extended cervical mediastinoscopy techniques. For “bulky” mediastinal tumors in close proximity to the anterior chest wall, an anterior mediastinotomy (Chamberlain’s procedure) is the procedure of choice (Fig. 171-4).
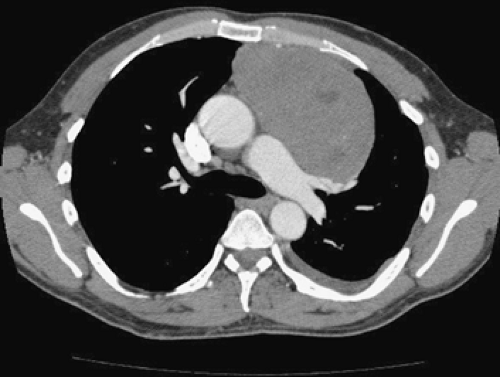 Figure 171-4. Anterior mediastinal mass abutting the anterior chest wall. This lesion is ideally suited for anterior mediastinotomy (Chamberlain’s procedure). |
 Figure 171-5. Video mediastinoscope. The use of video-assisted mediastinoscopy improves the ability to perform a more extensive nodal biopsy. (Copyright 2008, Karl Storz Endoscopy.) |
More recently, video-assisted mediastinoscopic lymphadenectomy (VAML) has demonstrated improved staging by increasing the number of removed lymph nodes.106,112,235,236,251 This translates into a sensitivity of 94%, specificity of 100%, accuracy of 98%, and negative and positive predictive values of 97% and 100% respectively. This technique uses a two-blade speculum that, when opened in the mediastinum, creates an operative field for bimanual surgery, improving the ability of the surgeon to perform a complete mediastinal lymph node dissection. VAML is particularly suited to identify minor N2 disease in patients eligible for neoadjuvant therapy. VAML is comparable to open mediastinal lymph node dissection in staging mediastinal lymph nodes in patients with lung cancer and can be performed safely after induction therapy. An additional advantage of VAML is that it makes teaching of mediastinoscopy easier for teachers and surgeons in training (Fig. 171-5). Disadvantages include a slightly increased rate of complications and a longer operative time as compared with standard cervical mediastinoscopy.
Because of the access and visual limitations of cervical mediastinoscopy and anterior mediastinotomy, VATS techniques have been explored as an adjunctive modality to evaluate mediastinal lymphadenopathy. VATS can provide a more comprehensive evaluation of the mediastinum when lymphadenopathy out of reach of the mediastinoscope is present.75 VATS conveys the ability to assess the entire ipsilateral mediastinum in greater scope than standard mediastinoscopy. It can also directly access the lower and posterior subcarinal region (station 7), periazygos area, and anterior (stations 5 and 6) and lower posterior (stations 8 and 9) mediastinal regions that are inaccessible to cervical mediastinoscopy. The VATS approach is especially useful when multiple sites within the mediastinum require biopsy, as demonstrated by Rendina and Landreneau.107,172 Additionally, VATS provides the opportunity to assess for mediastinal or chest wall invasion by the primary tumor and biopsy satellite nodules as well as to evaluate pleural effusions and perform pleurodesis if indicated, all at the time of mediastinal staging.75 Patients with a negative examination can proceed immediately to resection,
while those with positive findings are spared an unnecessary thoracotomy.107,172 The feasibility and accuracy of thoracoscopic lymph node staging in esophageal cancer have also been reported, alone or with the addition of laparoscopy.100,101,102,118,119 Unlike the case in lung cancer, in which mediastinoscopy has become a well-accepted procedure, the roles of thoracoscopy and laparoscopy in staging esophageal cancer remain to be realized. This technique may be especially applicable to patients suspected of having advanced locoregional disease in order to select them for clinical trials of preoperative chemotherapy, radiation therapy, or both. However, EUS remains the procedure of choice to sample upper abdominal and mediastinal lymph nodes in patients with esophageal cancer.
while those with positive findings are spared an unnecessary thoracotomy.107,172 The feasibility and accuracy of thoracoscopic lymph node staging in esophageal cancer have also been reported, alone or with the addition of laparoscopy.100,101,102,118,119 Unlike the case in lung cancer, in which mediastinoscopy has become a well-accepted procedure, the roles of thoracoscopy and laparoscopy in staging esophageal cancer remain to be realized. This technique may be especially applicable to patients suspected of having advanced locoregional disease in order to select them for clinical trials of preoperative chemotherapy, radiation therapy, or both. However, EUS remains the procedure of choice to sample upper abdominal and mediastinal lymph nodes in patients with esophageal cancer.
VATS is also quite useful in the evaluation of “primary” mediastinal lymphadenopathy associated with benign and malignant lymphoid conditions.95 The large tissue samples needed to evaluate lymph node architecture and to assess cellular clonal patterns characteristic of lymphoma are easily obtainable. In women, the anterior mediastinotomy incision through the breast is avoided with the use of the laterally orientated VATS intercostal access sites. At present, the author believes that the VATS approach is preferable to anterior mediastinotomy (i.e., Chamberlain’s procedure) for inaccessible lymphadenopathy by a standard cervical mediastinoscopy. However, the anterior mediastinotomy approach is preferred for the diagnosis of large anterior mediastinal mass lesions abutting the anterior chest wall (see Fig. 171-4). With careful planning and dissection, the procedure can be performed expeditiously under local anesthesia and intravenous sedation. In addition, chest tube insertion is not usually required.
Technical Considerations
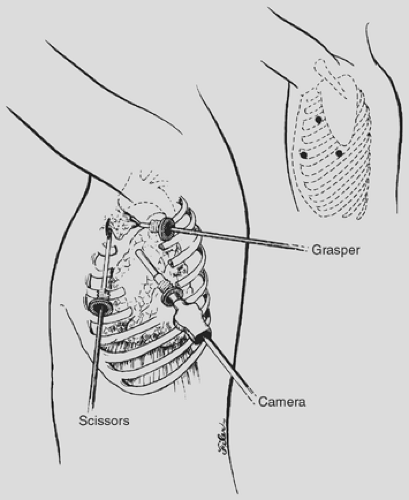
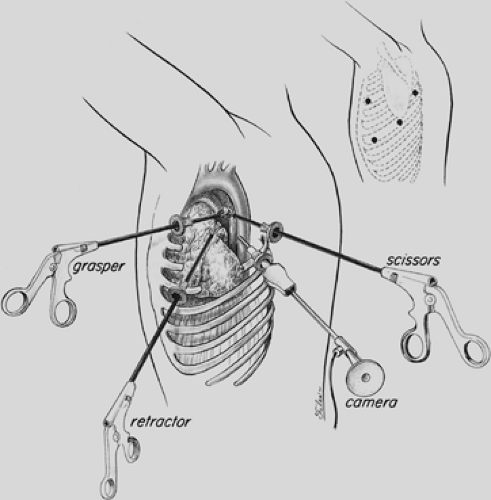
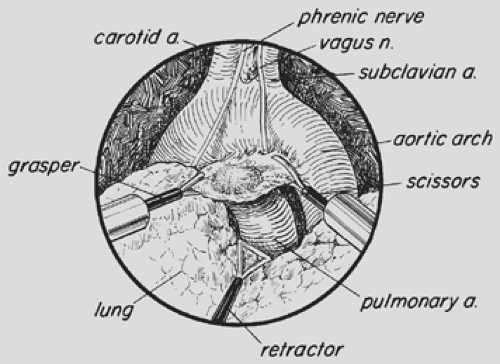
Double-lumen endotracheal intubation or lung isolation with a bronchial blocker and patient positioning are performed as detailed in Chapter 35. For the VATS approach to the anterior mediastinum (Fig. 171-6), the table is rotated posteriorly in order to expose more of the anterior chest. The ports are placed posterior to the midaxillary line and directed toward the anterior mediastinum with the surgeon standing at the back of the patient. For biopsy of paratracheal, subcarinal, and lower thoracic (esophageal, pulmonary ligament) lymph nodes, access port placement is similar to that utilized for VATS lobectomy. For routine VATS mediastinal lymph node dissection, the thoracoscope is introduced low in the chest (sixth to eighth intercostal space [ICSs]) along the midaxillary line for a panoramic view of the intrathoracic structures. Additional intercostal access sites are strategically positioned at the third to fourth ICS anterior to midaxillary line and the fifth ICS midaxillary line.95,107,108 A separate lung retractor may be used if visualization must be further enhanced, but this is usually not necessary. The intercostal approach to the right mediastinum is similar to that to the left, but for detailed evaluation of the aortopulmonary window (Figs. 171-7 and 171-8), the ICS access sites may be altered slightly (Table 171-2). Sharp dissection with the endoscopic Metzenbaum scissors (U.S. Surgical, Norwalk, CT; Snowden Pencer, Atlanta, GA) is used, with judicious applications of electrocautery. Alternatively, the ultrasonic coagulator can be used as the primary form of dissection and hemostasis. The locations of the phrenic, vagus, and recurrent laryngeal nerves are carefully identified, and dissection is directed to prevent injury to these structures.
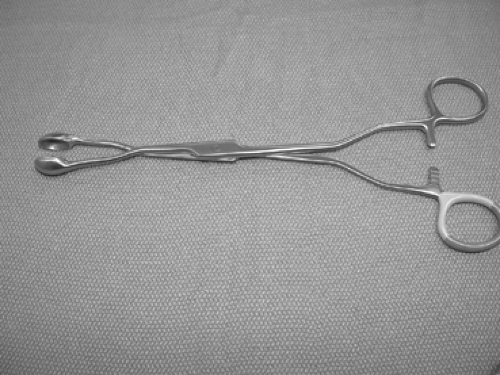
The mediastinal pleura is incised and the lymph nodes are identified and carefully dissected. Endoscopic clip ligature of vascular and lymphatic pedicles of the mediastinal lymph nodes is performed as needed. If the lymph nodes are large and matted, a mediastinoscopy cup forceps or a specially designed forceps can be utilized for biopsy (Fig. 171-9). The cut finger of a surgical glove can be utilized to extract small specimens. For dissection of the paratracheal lymph nodes, the mediastinal pleura above the hilum is opened and the lymph nodes dissected. On the right side, the entire fatty tissue containing the lymph nodes between the posterior aspect of the superior vena cava (anterior) and the trachea (posterior), the azygos vein (caudal), pericardium (medial), and the innominate artery (cephalad) can easily be dissected with electrocautery or ultrasonic coagulator. This technique is preferable to dissection of individual lymph nodes. If necessary, the azygos vein can be divided to allow access to lower paratracheal or tracheobronchial angle lymph nodes. On the left, care must be taken to avoid injury to the recurrent laryngeal nerve. The subcarinal lymph node packet is easily accessed on the right side but not on the left. However, by utilizing a flexible retractor for the esophagus and aorta, this can be easily accomplished. For dissection of the right subcarinal lymph nodes, the mediastinal pleura behind the lung is incised from the inferior pulmonary vein to the azygos vein. The right main bronchus is identified and careful dissection utilized to protect the esophagus posteriorly. The subcarinal lymph node packet is grasped with a ring forceps and dissected off the posterior pericardium. The node packet is elevated and dissection continued superiorly, taking care to avoid injury to the membranous portion of the airway. The right main, the left main bronchi and carina are carefully exposed and skeletonized. Small clips may be required to secure the bronchial arteries or lymphatic tributaries. On the left, the carina rests in front of the esophagus and aorta, making exposure a little more challenging. A liver retractor, as utilized in laparoscopic surgery, may help with exposure by displacing the esophagus and aorta posteriorly. With the lung retracted anteriorly, the pleura behind the lung is opened from the inferior pulmonary vein to the aortic arch, exposing the left main bronchus. A curved ring forceps is utilized to grasp the caudal aspect of the nodal packet and retracted cephalad toward the carina. Dissection with a combination of scissors and electrocautery or ultrasonic devices is performed, taking care to avoid injury to the airway. For exposure of level 5 and 6 mediastinal lymph nodes, the pleura overlying the aortopulmonary window is divided transversely, with care to avoid injury to the vagus, recurrent, and phrenic nerves. The superior edge of the pleura is retracted cephalad, exposing the underlying great vessels
and lymph nodes. A curved ring forceps is used to grasp the lymph node packet. Sharp dissection and judicious use of the ultrasonic dissector or electrocautery is made to dissect the nodal tissue off the underlying pulmonary artery (level 5) and aorta (level 6). Level 8 and 9 lymph nodes are easily dissected and removed during routine mobilization of the pulmonary ligament. A very thorough mediastinal lymph node dissection is possible, as demonstrated by Swanson and McKenna.133,209
and lymph nodes. A curved ring forceps is used to grasp the lymph node packet. Sharp dissection and judicious use of the ultrasonic dissector or electrocautery is made to dissect the nodal tissue off the underlying pulmonary artery (level 5) and aorta (level 6). Level 8 and 9 lymph nodes are easily dissected and removed during routine mobilization of the pulmonary ligament. A very thorough mediastinal lymph node dissection is possible, as demonstrated by Swanson and McKenna.133,209
Table 171-2 Strategic Intercostal Access Locations for Mediastinal Video-Assisted Thoracic Surgery | ||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||||||
Mediastinal Tumors
Primary mediastinal tumors are a heterogenous group of neoplastic, congenital, and inflammatory conditions. Neurogenic tumors, thymomas, and benign cysts account for approximately 60% of surgically resected lesions, while lymphoma, teratomas, and granulomatous diseases together comprise an additional 30%. Approximately two-thirds of all mediastinal tumors are benign. The likelihood of malignancy is primarily influenced by three factors: mass location, patient age, and the presence or absence of symptoms. Masses in the anterior compartment are more likely to be malignant (59%) than masses in the middle (29%) or posterior mediastinum (16%). Malignancy is more common in children than in adults. More than 75% of asymptomatic patients have benign lesions, while almost two-thirds of symptomatic patients have malignant tumors.
Video-Assisted Thoracic Surgical Management of Anterior Mediastinal Masses and Cysts
The differential diagnosis of anterior mediastinal masses includes lymphomas, thymic neoplasms or cysts, retrosternal goiter, mesenchymal tumors, germ cell tumors, and a variety of inflammatory, congenital, or developmental abnormalities. This topic has recently been reviewed.41,87,164,165,168,205,234 The VATS approach is well suited for resection of small benign tumors of the anterior mediastinum.1,24,27,34,37,167,177,178,245 However, with experience, complete resection of benign bulky mediastinal masses can be accomplished safely.59 The thoracic surgeon may also occasionally be asked to obtain a biopsy of a persistent or recurrent intrathoracic mass following treatment to assess response. In these cases, the surgical approach can present some technical problems owing to the reduction of the neoplastic mass and the reactive fibrosis induced by chemotherapy and radiation. In these cases, VATS offers the advantage of providing a wide field of view to select the most appropriate location for biopsy and simultaneous access to other sites of potential involvement, such as the lung and pleural surfaces. Robotic approaches have recently been described for the treatment of anterior mediastinal masses.10,187
Since most ectopic parathyroid glands or adenomas are found in close proximity to the thymus gland, these patients may be considered ideal candidates for VATS resection.5,2,103,166,215 Sestamibi scan, CT, and MRI are key to success by providing preoperative localization of the abnormality (Fig. 171-10). Parathyroid glands >1.5 cm are typically seen on CT scans, but smaller glands may be difficult to identify (Fig. 171-11). It must be further stressed that the VATS parathyroidectomy is planned as a directed resection following preoperative localization and not as a general exploration. To improve the detection of these small neoplasms and ensure resection, investigators have used radioisotope-navigated VATS and intraoperative parathyroid hormone testing to confirm cure and avoid additional explorations.153,154 VATS parathyroidectomy is primarily indicated for the more common anterior mediastinal parathyroid but can be an equally effective approach to the more unusual posterior mediastinal ectopic location. VATS provides excellent exposure to all mediastinal locations of ectopic parathyroid, providing near certain control of hyperparathyroidism in all
patients. This can be achieved with minimal morbidity and a very short length of hospitalization and recovery. Parathyroid cysts are also readily and increasingly excised using VATS.193
patients. This can be achieved with minimal morbidity and a very short length of hospitalization and recovery. Parathyroid cysts are also readily and increasingly excised using VATS.193
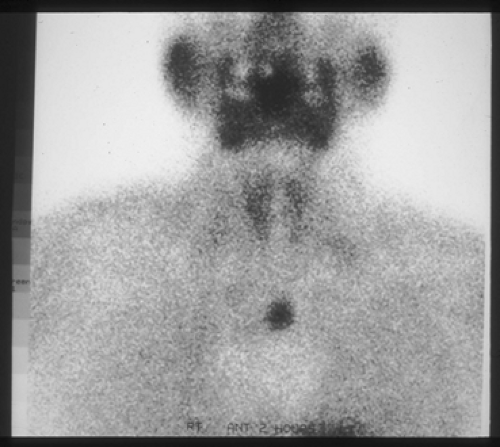 Figure 171-10. Sestamibi nuclear scan demonstrating uptake in the mediastinum. This parathyroid adenoma was resected thoracoscopically. |
Although VATS has been accepted as a reasonable approach to thymic cysts, the optimal approach to accomplish thymectomy remains a controversial issue.29,85,86,123,124,130,246 Accumulated clinical data have substantiated the utility of VATS in carefully selected patients with thymic disorders referred for surgical intervention, including thymic cysts, myasthenia gravis, and stage I to II thymoma.72,113,114,123,126,176,216,217,219,229,245,246 Careful patient selection for VATS thymectomy is vitally important, and considerable experience in the more elementary VATS procedures is recommended before thymic resection is attempted. Known malignant tumors and thymomas with preoperative evidence of local invasion should be resected through thoracotomy or sternotomy as deemed appropriate for the specific lesion. Furthermore, in order to minimize the likelihood of incomplete tumor resection, conversion to an open intervention is recommended when unexpected findings are appreciated during VATS. However, utilizing advanced thoracoscopic techniques, en bloc resection of the lung and pericardium can be safely accomplished.
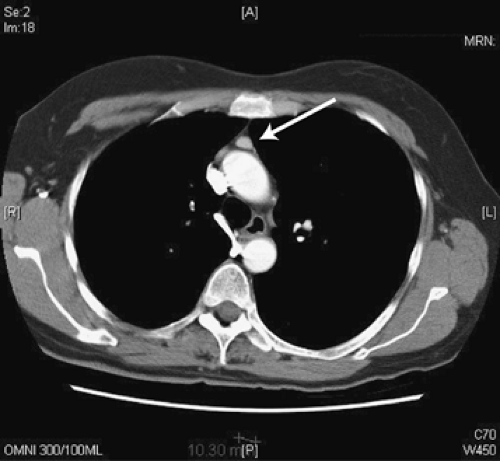
Landreneau109 first reported VATS resection of a stage I thymoma with subtotal thymectomy through the left chest (Fig. 171-12). This patient remains disease free nearly 18 years after complete VATS extirpation of the stage I thymoma. Nevertheless, complete tumor removal and total thymectomy (thymothymectomy) is the appropriate surgical treatment for thymoma with or without myasthenia gravis. Recent reports support the application of VATS as a valid procedure for patients with stage I and II thymoma.22,23 Factors associated with complete resection include a fat plane between the tumor and vital structures, unilateral tumor predominance, tumor encapsulation, existence of residual normal-appearing thymic tissue, and no mass compression effect on neighboring structures.23 Complete resection of the tumor and involved structures with negative margins (R0) is the goal. Operative experience has demonstrated that a “total” anatomic thymectomy can be performed by VATS21,23,50,121,124,127,189,194,238,245,246,247,252 (Fig. 171-13). Mantegazza127 and others121,194 recommend a combination of cervical and bilateral thoracoscopic approach to achieve a video-assisted thoracoscopic extended thymectomy without sternotomy. Although VATS thymectomy has been criticized by some as an “incomplete” resection, midterm data following VATS thymectomy compare well with historical series with transsternal thymectomy and even “maximal” thymectomy.127,139,185,219,238 Longer-term follow-up will be necessary before it can be stated that VATS thymectomy is as effective as sternotomy in accomplishing thymectomy for the management of myasthenia gravis or stage I to II thymomas; however, preliminary results are encouraging.22,23,113,229
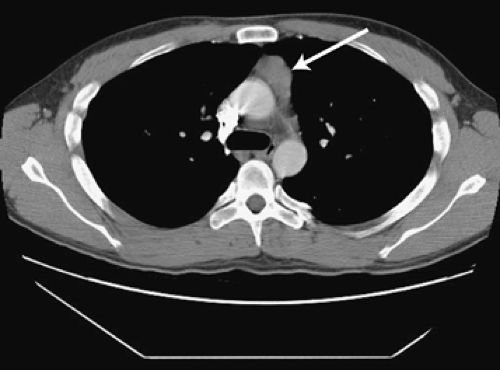 Figure 171-12. CT scan of the chest demonstrating a soft tissue mass in the anterior mediastinum (arrow). This pathologically stage I thymoma was resected thoracoscopically. |
Technical Considerations
Recent reports advocate the use of multidetector CT with three-dimensional reconstruction to identify the venous drainage pattern of the thymus in anticipation of the VATS resection.148,198 The information obtained from this study has the potential to make the thoracoscopic resection safer by clearly identifying the anatomy of the thymic veins draining into the brachiocephalic veins, thus reducing the incidence of intraoperative bleeding. Single-lung ventilation facilitates the procedure, although resection can also be accomplished with mild CO2 insufflation or during spontaneous respiration under intravenous sedation. The general VATS surgical routine and intercostal access is the same as outlined for other anterior mediastinal pathology.95 Alternatively, the patient may be positioned supine with the ipsilateral arm slightly below the level of the table to allow unimpeded access to the hemithorax, or with the arms raised over the head and flexed at the elbow (Fig. 171-14). Three 5-mm ports are utilized around the breast tissue. A 30-degree thoracoscope, an endoscopic ultrasonic shears, and a tissue grasper are required. Artificial induction of pneumomediastinum by injection of air behind the manubrium may facilitate thoracoscopic dissection in the anterior mediastinum.224 Clinical experience has led to several technical refinements to the approach for VATS thymectomy. Most importantly, some surgeons have adopted a right-sided approach in lieu of a left-sided approach, as described by
Mineo139 and Landreneau.109 However, others continue to advocate a left-sided approach if the thymic pathology is largely within the left chest. Alternatively, a bilateral approach has also been suggested to perform an extended thoracoscopic resection. Although the right-sided approach makes evaluation of the aortopulmonary window difficult for ectopic thymic tissue, there is excellent visualization of the junction of the innominate vein and the superior vena cava during the course of the dissection, as described by Yim.248 This exposure facilitates the dissection while avoiding troublesome bleeding from venous tributaries at this region. A combined transcervical–subxiphoid thoracoscopic approach has also been described.252 The operation is begun by performing a quick survey of all mediastinal and pleural surfaces to exclude unexpected pathology. The phrenic nerve is carefully identified and the mediastinal pleura is incised just anterior to the phrenic nerve near the diaphragm utilizing the ultrasonic shears or electrocautery device on low setting to avoid injury to the phrenic nerve (Fig. 171-16). The dissection progresses cephalad to the thoracic outlet until the innominate vein is reached. The dissection then continues behind the sternum, starting caudally and progressing cephalad toward the thoracic outlet to join with the prior plane of dissection near the innominate vein. By gently pulling on the thymic tissue, the plane of dissection is clearly identified, making sure to avoid injury to the internal mammary vessels. Transmediastinal dissection is performed behind the sternum until the contralateral pleura is exposed and incised. Tension on the thymus is avoided when dissection proceeds to the superior aspect of the thymus to avoid avulsion of the thymic veins (of Keynes) or injury to the innominate vein. The thymic veins are identified and coagulated or doubly clipped on the side of the innominate vein before their transection. Ultrasonic devices are safe and reliable during this part of the dissection, providing excellent hemostasis. The cervical horns of the thymus are gently retracted, dissected out from the neck, and divided, taking care to skeletonize and protect the innominate vein (Fig. 171-17A,B). The thymus is then dissected from the pericardium in the avascular plane until complete removal. Alternatively, contralateral access is performed to achieve an extended thoracoscopic resection. The specimen is placed in a bag and removed after enlarging one of the access incisions. Care in handling the tumor, if present, is paramount to avoid penetration of the capsule and potential contamination. Conversion to thoracotomy or sternotomy when intraoperative evidence of local invasion or other signs of malignant disease are present is appropriate, although en bloc resection of lung and pericardium can be performed with VATS.22,23 Except for ectopic parathyroid adenomas, a complete resection of the thymus is recommended for all other mass lesions of the thymus or in the presence of myasthenia gravis. A single small chest tube is placed transmediastinally to reexpand both lungs. Most patients can be discharged home within 24 hours. Video-assisted thymectomy is covered in more detail in Chapter 184. A robotic approach to anterior mediastinal pathology has been reported; results in myasthenia gravis patients are comparable to those reported by VATS and open approaches at the same length of follow-up.18,169 Robotic surgery is discussed in Chapter 34.
Mineo139 and Landreneau.109 However, others continue to advocate a left-sided approach if the thymic pathology is largely within the left chest. Alternatively, a bilateral approach has also been suggested to perform an extended thoracoscopic resection. Although the right-sided approach makes evaluation of the aortopulmonary window difficult for ectopic thymic tissue, there is excellent visualization of the junction of the innominate vein and the superior vena cava during the course of the dissection, as described by Yim.248 This exposure facilitates the dissection while avoiding troublesome bleeding from venous tributaries at this region. A combined transcervical–subxiphoid thoracoscopic approach has also been described.252 The operation is begun by performing a quick survey of all mediastinal and pleural surfaces to exclude unexpected pathology. The phrenic nerve is carefully identified and the mediastinal pleura is incised just anterior to the phrenic nerve near the diaphragm utilizing the ultrasonic shears or electrocautery device on low setting to avoid injury to the phrenic nerve (Fig. 171-16). The dissection progresses cephalad to the thoracic outlet until the innominate vein is reached. The dissection then continues behind the sternum, starting caudally and progressing cephalad toward the thoracic outlet to join with the prior plane of dissection near the innominate vein. By gently pulling on the thymic tissue, the plane of dissection is clearly identified, making sure to avoid injury to the internal mammary vessels. Transmediastinal dissection is performed behind the sternum until the contralateral pleura is exposed and incised. Tension on the thymus is avoided when dissection proceeds to the superior aspect of the thymus to avoid avulsion of the thymic veins (of Keynes) or injury to the innominate vein. The thymic veins are identified and coagulated or doubly clipped on the side of the innominate vein before their transection. Ultrasonic devices are safe and reliable during this part of the dissection, providing excellent hemostasis. The cervical horns of the thymus are gently retracted, dissected out from the neck, and divided, taking care to skeletonize and protect the innominate vein (Fig. 171-17A,B). The thymus is then dissected from the pericardium in the avascular plane until complete removal. Alternatively, contralateral access is performed to achieve an extended thoracoscopic resection. The specimen is placed in a bag and removed after enlarging one of the access incisions. Care in handling the tumor, if present, is paramount to avoid penetration of the capsule and potential contamination. Conversion to thoracotomy or sternotomy when intraoperative evidence of local invasion or other signs of malignant disease are present is appropriate, although en bloc resection of lung and pericardium can be performed with VATS.22,23 Except for ectopic parathyroid adenomas, a complete resection of the thymus is recommended for all other mass lesions of the thymus or in the presence of myasthenia gravis. A single small chest tube is placed transmediastinally to reexpand both lungs. Most patients can be discharged home within 24 hours. Video-assisted thymectomy is covered in more detail in Chapter 184. A robotic approach to anterior mediastinal pathology has been reported; results in myasthenia gravis patients are comparable to those reported by VATS and open approaches at the same length of follow-up.18,169 Robotic surgery is discussed in Chapter 34.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree