Video-Assisted Esophageal Resection
Christopher R. Morse
Manisha R. Shende
James D. Luketich
Transhiatal esophagectomy and Ivor Lewis esophagogastrectomy are the two most commonly performed operations for esophageal carcinoma. Transhiatal esophagectomy is performed with a midline abdominal incision and blunt dissection of the thoracic esophagus through the esophageal hiatus. Following the creation of a gastric tube, the gastric conduit is pulled through the esophageal bed and a cervical esophagogastric anastomosis is performed. An Ivor Lewis esophagectomy is performed through an upper midline abdominal incision with construction of the gastric conduit. A right thoracotomy is performed with dissection and removal of the thoracic esophagus and construction of an intrathoracic esophagogastric anastomosis. These operations are frequently associated with significant morbidity and mortality. In a report summarizing nationwide statistics, the mortality rates from esophagectomy ranged from 8% in high-volume centers to as high as 23% in low-volume centers.3 Furthermore, esophagectomy is often performed in elderly patients who have medical comorbidities including respiratory and cardiovascular disease. In an effort to limit the physiologic stress and possibly reduce the morbidity associated with open esophagectomy, minimally invasive surgical approaches to esophagectomy have been developed.
Since the introduction of laparoscopic fundoplication in 1991, improvements in instrumentation and optics have allowed the development of minimally invasive approaches to esophageal diseases that were traditionally managed by open operation.9 These include achalasia, giant paraesophageal hernia, and other complex esophageal disorders.14,19,20,21,24 This paradigm shift has been driven by advanced minimally invasive surgical technology and the observation that minimally invasive surgery is associated with equal efficacy, less pain, and an earlier return to work as compared with open surgery. An open approach is still the standard of care, however, for patients with esophageal cancer, because of concerns that (a) minimally invasive surgery may not be equivalent in terms of nodal clearance and the oncologic completeness of resection, (b) the complexity of the procedure would be prohibitive in most centers, and (c) a minimally invasive approach may not have a measurable impact on morbidity. However, a number of recent series reporting the outcomes of minimally invasive esophagectomy (MIE) are challenging these concerns. Although no randomized studies have been performed, in this chapter we will present our results and that of others showing that MIE is associated with oncologic outcomes comparable to those of open surgery, that advanced fellowship training produces surgeons capable of performing minimally invasive esophagectomy, and that MIE is associated with lower rates of complications and mortality.
Origins of a Minimally Invasive Approach to Esophageal Resection
Initial attempts at minimally invasive esophageal resections were hybrid operations consisting of some steps of the operation performed in a minimally invasive fashion and others performed through open incisions. For example, thoracoscopy and esophageal mobilization were introduced in an attempt to reduce the respiratory morbidity associated with a right thoracotomy.1,8 In these early reports, thoracoscopic esophageal mobilization still required a standard midline laparotomy for construction of the gastric conduit in combination with gastric pull-up and construction of a cervical esophagogastric anastomosis. Alternatively, another hybrid technique for esophagectomy was described with the laparoscopic construction of a gastric conduit followed by a right thoracotomy for removal of the thoracic esophagus and construction of an intrathoracic esophagogastrostomy.5,11,12 These hybrid approaches to esophagectomy utilized minimally invasive techniques but continued to require either a thoracotomy or laparotomy and failed to show any consistent advantages over conventional open esophagectomy. As Law et al. reported,16 it is difficult to draw any conclusions about advantages of any particular hybrid approach, given that morbidity and mortality rates were no different from the conventional approach.
In 1995, DePaula and colleagues were the first group to report a small series of laparoscopic transhiatal esophagectomy performed totally via laparoscopy.11 Their approach was a laparoscopic construction of the gastric conduit followed by laparoscopic transhiatal mobilization of the mediastinal esophagus. A neck incision was performed for construction of an esophagogastric anastomosis. Subsequently in 1998, Luketich et al. reported a thoracoscopic esophageal mobilization followed by laparoscopic construction of the gastric conduit, gastric pull-up, and neck anastomosis.20 A minimally invasive Ivor Lewis technique was reported in 1999 by Watson et al., who described the laparoscopic construction of the gastric conduit followed by thoracoscopic esophagectomy with construction of an intrathoracic esophagogastric anastomosis.27
Surgical Techniques
Minimally Invasive Ivor Lewis
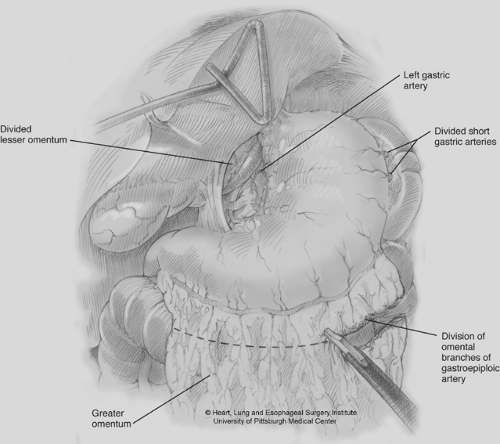
The patient is initially positioned supine and a double-lumen endotracheal tube is placed for eventual thoracoscopy. On-table esophagogastroscopy is performed to assess precise tumor location and any extension proximally or distally (onto the cardia of the stomach). The suitability of the stomach as a gastric conduit is also evaluated. The surgeon remains on the right side while the assistant is positioned to the patient’s left. Five abdominal ports are used for the gastric mobilization (Fig. 140-1). The initial port is placed via an open technique and, in contrast to a hiatal hernia repair, the two midclavicular ports are placed slightly lower to assist with gastric mobilization. After an initial inspection of the peritoneal surfaces and the liver to rule out any metastatic disease, the gastrohepatic omentum is opened. The left gastric artery/vein pedicle is identified and the celiac nodes are examined. If there are any enlarged nodes suspicious for metastatic implants, they are dissected out and sent for frozen section analysis. If the nodes are not worrisome or return negative, the right crus is dissected free, mobilizing the lateral aspect of the esophagus. The dissection is then carried anteriorly and superiorly, over the esophagus, mobilizing the anterior hiatus. Continuing this dissection toward the left crus, the fundus of the stomach begins to be mobilized. We do not completely divide the phrenoesophageal ligament until the conclusion of laparoscopy, as this allows for the maintenance of pneumoperitoneum. After creating a retroesophageal window by completing the dissection of the inferior aspect of the right crus, attention is turned to the gastrocolic omentum. By carefully retracting the antrum of the stomach, a window is created in the greater omentum, allowing access to the lesser sac. This is done while carefully preserving the right gastroepiploic vessel. Dissection is carried along the greater curve of the stomach until the end of the gastroepipoloic arcade is reached. The short gastric vessels are taken with a combination of the ultrasonic shears (Autosonix, Covidien, Mansfield, MA; Harmonic Scapel, Johnson & Johnson, Piscataway, NJ) and the LigaSure device (LigaSure, Valleylab, Boulder, CO). Occasionally clips will be used to control large, short gastric vessels. With the greater curve of the stomach mobilized, the fundus is rotated toward the patient’s right shoulder, exposing the retrogastric attachments. These are dissected free until the left gastric artery and vein are encountered. The retrogastric attachments are also divided towards the hiatus, completely mobilizing the fundus and the distal esophagus. The mobilization of the stomach is then carried back toward the pyloroantral region, where the dissection must be meticulous. An injury to the gastroepiploic arcade or the gastroduodenal artery may render the gastric conduit unusable. There are often significant retroantral and periduodenal adhesions that must be taken to allow for adequate mobilization of the stomach into the chest. The pylorus is considered adequately mobilized when it is able to reach the right crus under no tension (Fig. 140-2).
Once the stomach and celiac lymph nodes are completely mobilized, the left gastric artery and vein are divided with a vascular load on the Endo GIA stapler (Autosuture, Covidien, Mansfield, MA). This is done by approaching the pedicle from the lesser curve. It is important that the pedicle be dissected completely clean with all celiac nodes swept up into the specimen. Once the pedicle is divided, the distal esophagus, the gastric fundus and antrum should be completely mobilized.
The gastric tube is created prior to completion of the pyloroplasty and placement of the jejunostomy tube, as this provides time to assess the conduit before bringing it into the chest. The initial staple line in creating the gastric conduit is a vascular load across the vessels on the lesser curve (but not onto the gastric antrum). The initial 5/12-mm right midclavicular port is changed to a 15-mm port to allow for the placement of a 4.8-mm Endo GIA stapler. An additional 12-mm port is placed in the right lower quadrant to assist with the creation of the gastric tube. During this step, it is important to have the first assistant grasp the tip of the fundus along the greater curve and gently stretch it toward the spleen, while a second grasper is placed on the antral area with slight downward retraction applied. This places the stomach on a slight stretch and assists in creating a straight staple line. The staple line should parallel the gastroepiploic arcade. Initially, using 4.8-mm staple loads, the stomach is divided across the antrum, which is generally quite thick and requires the larger stapler loads for secure closure. The goal is to create a conduit that is 5 cm wide (Fig. 140-3). Early in our experience, we discovered an increase in gastric tip necrosis and anastomotic leaks with a conduit 3 or 4 cm wide. Once the antrum is divided, the left midclavicular port is changed back to an 11-mm port and the fundus is divided using a 3.5-mm stapler. The graspers are readjusted as the fundus is divided, again to keep the stomach on stretch. If there is any concern about extension of tumor onto the gastric cardia, a wider margin is left in this region.
It has not been our practice to oversew the staple line. The right gastric vessels are preserved.
It has not been our practice to oversew the staple line. The right gastric vessels are preserved.
After the mobilization and creation of the gastric conduit, a pyloroplasty is performed. Initially, holding sutures are placed on the superior and inferior aspect of the pylorus. Once the pylorus has been placed on stretch, it is opened with ultrasonic shears. The pyloroplasty is then closed in a Heineke–Mikulicz fashion using interrupted sutures. This usually takes three to four stitches (Fig. 140-4). At the conclusion of the abdominal portion of the operation, the pyloroplasty is covered with omentum. In our experience, a laparoscopic pyloromyotomy is difficult to perform.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree