Ventricular Tachycardia
Ventricular tachycardias (VTs) include a spectrum of arrhythmias that range from nonsustained asymptomatic VT to a sustained arrhythmia that results in a cardiac arrest. VTs are defined by morphology and duration. They may be uniform in morphology (monomorphic) or polymorphic. They may be unsustained or sustained (defined arbitrarily as >15 to 30 seconds) unless cardioverted sooner because of hemodynamic intolerance). Sustained monomorphic VT most often occurs in the setting of prior myocardial infarction (MI). Sustained VTs (polymorphic or monomorphic) may also be seen in patients without structural heart disease or in a variety of disorders including cardiomyopathies, valvular disease, drug toxicity, metabolic disorders, and ion channelopathies (see Fig. 10-1).
Nonsustained VTs occur in many people without known heart disease. Although polymorphic VT can be seen in acute ischemia, this is rare except with associated marked ST segment changes (see Fig. 10-2). Most often these VTs occur in the setting of prior infarction or cardiomyopathy with small scars (i.e., insufficient slow conduction for production of monomorphic VT) or in normal ventricles due to functional reentry (e.g., Brugada syndrome, drug effect, long QT syndromes) which may be initiated by early afterdepolarizations (EADs) (see the following text) or catecholamine-induced triggered activity.
Identifying the Mechanisms and Substrate of Ventricular Tachycardia
Mechanisms of Ventricular Tachycardia
Reentry: It is the most common mechanism of paroxysmal, sustained monomorphic VT in the setting of structural heart disease associated with scar, of which coronary artery disease (CAD) is most common. Reentry is characterized by:
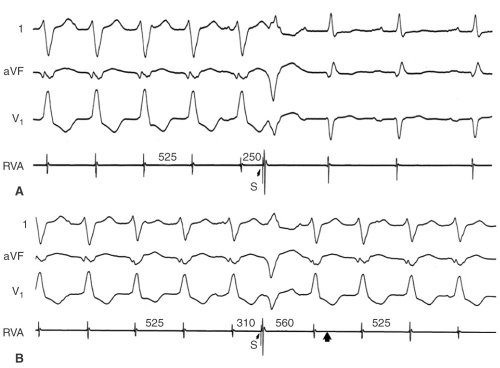
Initiation and termination by timed extrastimuli which are often site-specific (e.g., right ventricular apex [RVA], outflow tract, or left ventricle) (see Fig. 10-3)
Timed ventricular extrastimuli, which reset the tachycardia with fusion (Fig. 10-3)
Entrainment of the tachycardia (see Fig. 10-4)
Triggered rhythms due to delayed afterdepolarizations (DADs) are catecholamine sensitive and often occur during exercise. Such rhythms arise in otherwise normal tissue (Purkinje fibers, right ventricle [RV] and left ventricular outflow tract [LVOT], aorta in the right and left coronary cusp, and the mitral annulus), or in recently infarcted or reperfused and stunned myocardium.
Triggered VT
Can be initiated by pacing more easily than by timed extrastimuli.
Are more difficult to initiate on repeated attempts.
Exhibit overdrive acceleration.
Cannot be entrained or reset with fusion.
Can be terminated by vagal maneuvers, adenosine, and by Na channel, β-adrenergic receptor or Ca channel blocking drugs.
Digitalis-induced VT is also due to DADs, which are thought to be responsible for catecholamine-induced polymorphic tachycardias. Therefore, DADs
can lead to monomorphic (e.g., RV/LVOT VTs) or polymorphic (e.g., ryanodine receptor defect) VTs.
can lead to monomorphic (e.g., RV/LVOT VTs) or polymorphic (e.g., ryanodine receptor defect) VTs.
Triggered rhythms initiated by EAD lead to polymorphic VTs associated with congenital and acquired long QT syndromes. These may also be seen in situations when there is calcium overload associated with short action potentials. This may explain polymorphic VTs following carotid sinus pressure or adenosine termination of supraventricular tachycardia (SVT).
Automatic VT: These are neither initiated nor terminated by programmed stimulation. They are seen in diseased tissue in which depolarized myocardial fibers develop phase 4 depolarization. Depending on the degree of depolarization overdrive suppression may or may not be seen. This mechanism may be seen after MI.
Substrate of Ventricular Tachycardia
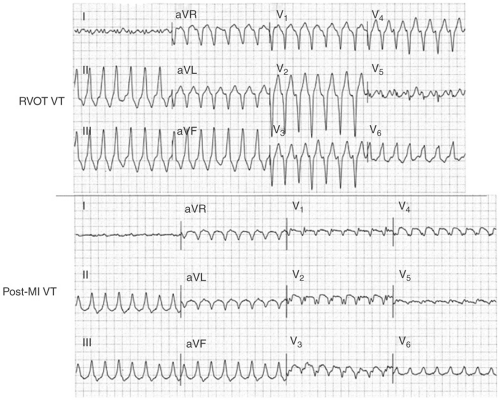
The electrocardiogram (ECG) is useful in defining the underlying pathology. VT in patients with normal hearts (idiopathic VT) is characterized by tall, smoothly inscribed QRS complexes, whereas VTs in patients with diseased myocardium, particularly those with extensive scarring, have smaller, broader, and notched or splintered QRS’ (see Fig. 10-5). Idiopathic VTs can have rapid initial forces whereas those VTs arising in scar have slower initial forces. A QS complex has no diagnostic value for underlying pathology. It can be seen in infarct-related VT, VT in cardiomyopathy, or even idiopathic VT arising on the epicardium (see the following text). A qR or QR complex in two anatomically adjacent leads is almost diagnostic of infarction. In many cases the infarct is more obvious during VT than in sinus rhythm.
The substrate of VT can be more accurately assessed by mapping the right and left ventricles during sinus rhythm. The normal heart has bipolar electrograms (EGMs) that are biphasic or triphasic, 1.5 mV (Carto) or 3 mV (standard Bard catheter) in amplitude, and which are <70 msec in duration. Abnormal EGMs can be classified as follows:
Low amplitude (<1.5 mV Carto)
Split (30 to 50 msec isoelectric interval)
Late (inscribed after the QRS)
Fractionated (low amplitude with multiple component)
Prior infarction scar is associated with low-amplitude signals ≤0.5 mV with a variable number of late, split, and fractionated signals. In patients with idiopathic cardiomyopathy the endocardium is less abnormal, with a smaller area of low-amplitude potentials, and a smaller percentage of split, late, and fractionated signals which are more frequent near the annuli. Such findings are more common on the epicardium in these patients. Arrhythmogenic RV
dysplasia is characterized by abnormal EGMs, primarily at the free wall of the RV. In approximately 15% to 20% of infarcts (primarily inferior) epicardial scar is more marked than endocardial scar.
dysplasia is characterized by abnormal EGMs, primarily at the free wall of the RV. In approximately 15% to 20% of infarcts (primarily inferior) epicardial scar is more marked than endocardial scar.
Patients with sustained monomorphic VT have a greater number of abnormal EGMs than those with ventricular fibrillation (VF) or nonsustained VT, regardless of whether prior infarction or idiopathic cardiomyopathy is present. Because the abnormal EGMs are associated with slow conduction, these areas, not surprisingly, are the source of reentrant arrhythmias. Arrhythmias in apparently normal areas are more frequently due to triggered activity or automaticity. Such mechanisms can also be operative in diseased hearts.
Differentiation of Ventricular Tachycardia from Supraventricular Tachycardia with Aberration
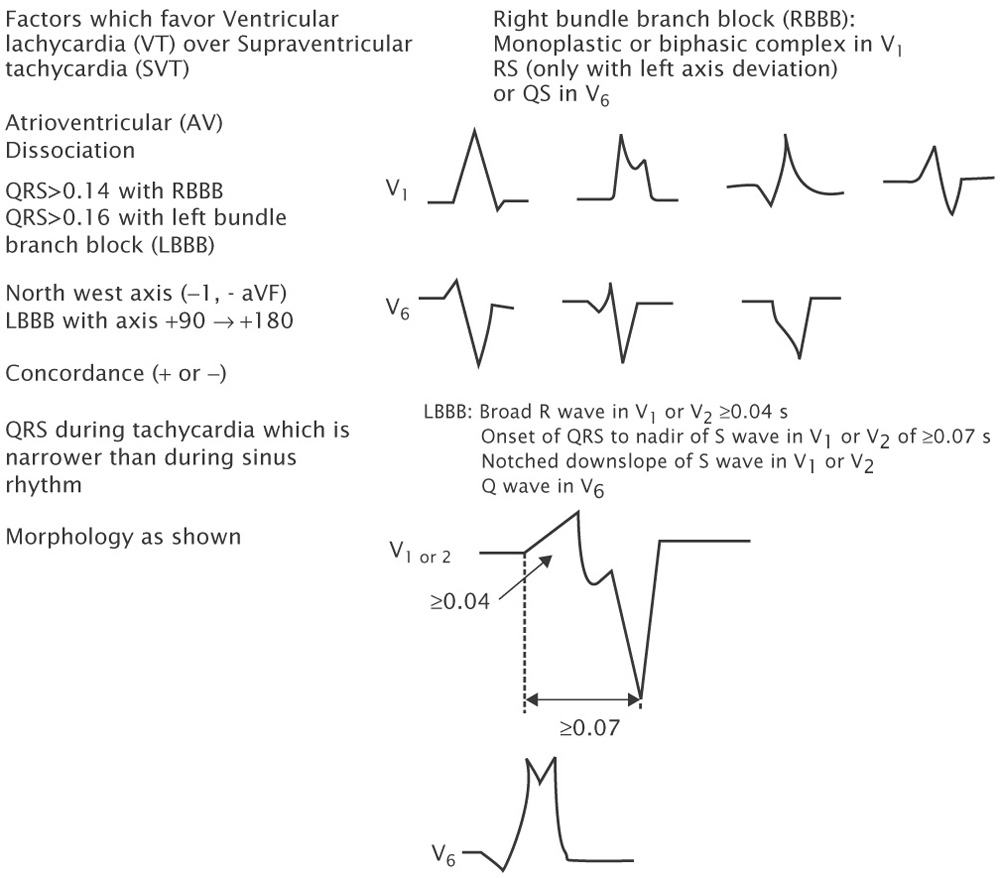
Several ECG criteria have been proposed to diagnose VT (see Table 10-1). Although none are perfect, several generalizations can be made:
Table 10-1 Electrocardiographic Criteria for Diagnosis of Ventricular Tachycardia | |
|---|---|
|
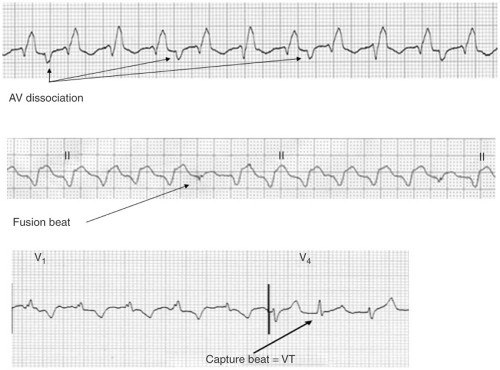
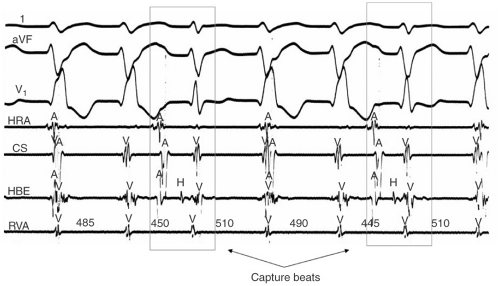
A-V dissociation, particularly when demonstrated by the presence of fusion and/or capture beats is virtually diagnostic of VT (see Figs. 10-6 and 10-7). Unfortunately capture beats are very uncommon and at very fast rates P waves may be difficult to see. Moreover one to one ventriculoatrial (VA) conduction may be seen in VT (usually at rates <200 bpm).
QRS width is useful in the absence of antiarrhythmic drugs or preexistent bundle branch block (BBB). Right bundle branch block (RBBB) aberration does not increase the QRS duration >0.14 seconds even with hypertrophy. Left bundle branch block (LBBB), which can produce a QRS of 0.14 seconds in a normal heart, can cause the QRS to reach 0.16 seconds in hypertrophy. Therefore, a RBBB-like complex >0.14 seconds and LBBB-like complex >0.16 seconds in the absence of drugs favors the diagnosis of VT. Of note somewhere between 2% and 5% of VTs have QRS′ ≤120 msec.
Axis may also be useful. An axis in the “northwest” quadrant is almost always VT in adults because no form of aberration has such a vector (see Fig. 10-8). In someone with a normal QRS in sinus rhythm, a LBBB-like wide complex tachycardia with a right axis (+90 to +180) is always VT because activation in LBBB aberration always goes from right to left.
Concordance: If all the precordial leads are positive (R) or negative (QS) the rhythm is very likely VT (see Fig. 10-9).
V1-V2 morphology: In RBBB-like tachycardias an RsR’ or rsR’ in V1 favors SVT, whereas a monophasic R, Rr’ (Fig. 10-8), qR, or RS favors VT. In RBBB aberration the initial forces of the QRS are the same as in the narrow complex sinus rhythm. In LBBB-like tachycardias V1 and V2 require analysis because the initial forces in V1 are often isoelectric. An R wave in V1 or V2 ≥40 msec favors VT. In addition, if the time from the onset of the QRS to the nadir of the S wave in V1 or V2 is ≥70 msec, VT is likely in the absence of Na channel blocking agents.
V6 may be useful in diagnosis as well. A QS or rS favors VT in RBBB-like tachycardias, although this can be influenced by axis (it is almost always seen in VT with left axis deviation, but is seen in only approximately 50% of
VT with a normal axis, even in the same patient) (Fig. 10-8). In LBBB-like tachycardias, a qR or QS is highly predictive of VT.
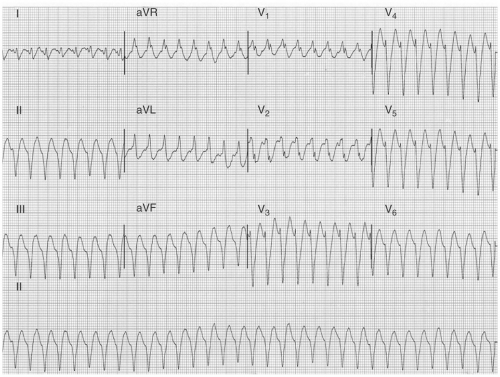
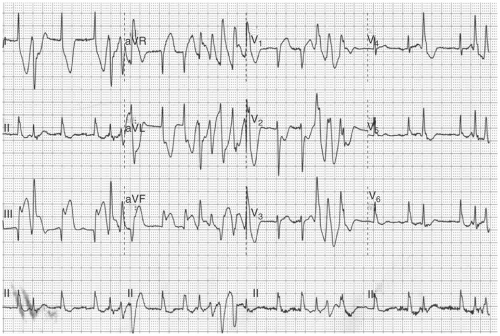
FIGURE 10-8. Ventricular tachycardia from the inferior wall of the left ventricle. The QRS complex has an Rsr’ right bundle branch block morphology, a northwest axis, and a Q wave in V6.
If BBB or an intraventricular conduction disturbance (IVCD) is present in sinus rhythm, a wide QRS that is narrower must be VT because intraventricular conduction stays the same or gets slower at increasing rates.
Role of the Electrophysiology Laboratory in the Evaluation and Therapy of Ventricular Tachycardia
The electrophysiology (EP) laboratory is useful for establishing the diagnosis and mechanism of VT, as well as localizing the site of origin (or exit) of the VT circuit and developing therapy. It is most useful for monomorphic VTs as polymorphic VTs do not pose a diagnostic challenge electrocardiographically, and are difficult to study by electrophysiology study (EPS). In these VTs the underlying substrate may be identified by mapping. In cases where a scar is present, but small, addition of an Na channel blocker (e.g., intravenous [IV] procainamide) may change the rhythm to a monomorphic VT that is inducible and able to be pace terminated, suggesting a reentrant arrhythmia. Catecholamine infusion may induce monomorphic as well as polymorphic VTs.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree