Fig. 13.1
An ECG of ventricular tachycardia presenting in a 3-day-old with mild LV dysfunction. The tachycardia exhibits a left bundle branch block with an inferior axis suggesting origin of the tachycardia from the right ventricular outflow tract. This VT has 1:1 VA conduction, with the P waves most easily seen in V1 and V2
Atrial (A) and ventricular (V) dissociation with more ventricular complexes than atrial complexes (Fig. 13.2).
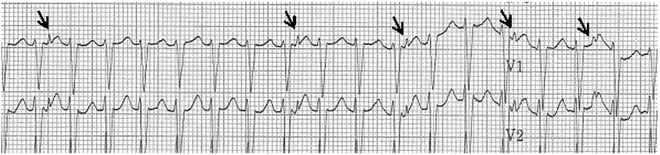

Fig. 13.2
An ECG rhythm strip on the patient from Fig. 13.1 after an adenosine bolus. Note the loss of retrograde VA conduction with adenosine administration confirming VT. Arrows indicate the retrograde P waves
A rate greater than 25 % above that of normal sinus rhythm, or greater than 120 bpm in adults.
However, these criteria are not necessarily applicable to the young patients. VT in infants may be associated with QRS durations of only ~80 ms, requiring comparison of the VT-QRS duration and morphology to age-appropriate norms. Although retrograde ventricular-atrial dissociation during VT is virtually diagnostic for VT, a good number of patients may display 1:1 (Fig. 13.1) or retrograde Wenckebach conduction during VT indicating that VA dissociation is not a prerequisite for VT. Because AV dissociation may be difficult to determine, a long rhythm strip may assist in its identification. Adenosine may be given to transiently block retrograde AV nodal conduction to assist in making the diagnosis (Fig. 13.2). Additionally, capture and fusion beats may be identified by a sinus-driven beat conducting antegrade through the AV node and influencing the initial deflection (fusion beat) or normalizing (capture beat) the QRS complex. When this is seen, it suggests a ventricular origin of the wide complex arrhythmia (Fig. 13.3). The likelihood of observing these beats is inversely proportional to the VT rate. In asymptomatic patients with a ventricular rate within ~125 % of the sinus rate an accelerated idioventricular rhythm (AIVR) is likely and is associated with a benign course. AIVR is somewhat more common in infants and children than adults.

Fig. 13.3
A Holter monitor strip showing lack of VA conduction and fusion complexes (beat three and others) and sinus captures (beat six and others)
In some cases, it may be difficult to distinguish VT from an atrial-driven rhythm with aberrancy of QRS conduction. To improve the diagnostic sensitivity suggested expanded criteria may be employed:
A QRS complex with both an initial and a terminal conduction delay Fig. 13.4a
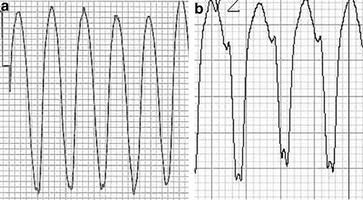
Fig. 13.4
(a) A 16-year-old male with VT associated with ARVC showing the initial and terminal conduction delay from V1. (b): A notched S negative deflection in a patient with an LBBB appearance VT from V2 from a different patient
A shift in the QRS axis by more than 40° from baseline
Fusion beats or capture beats (Fig. 13.3)
A QRS axis between −90 and 180°
QRS duration greater than 0.14 s (with RBBB) or 0.16 s (LBBB)
A taller initial (leftward) peak in the QRS complex in a right bundle branch complex
A delayed S wave nadir with a notched S down stroke in a left bundle branch complex (Fig. 13.4b)
Though many of these criteria may be useful, they remain imperfect as the sensitivity may be below 50 %. Therefore, care should be used when applying them, and other potential criteria to wide QRS tachyarrhythmia diagnosis.
Often vector analysis can be employed to discern the origin of the VT. Because the wave front moves away from the point of origin, an ECG complex in the precordial leads that has predominately leftward forces (with an LBBB appearance) predicts an origin from the right ventricle and, conversely, predominately rightward forces (with an RBBB appearance) predicts an origin from the left ventricle. Further, a frontal plane (from the limb leads) superior QRS axis of the VT suggests origin from the inferior ventricular segments, and conversely, a frontal plane inferior QRS axis suggests origin of the VT from the base of the heart, usually the right ventricular outflow tract or coronary cusps.
Differential Diagnosis of Wide QRS Tachycardia
A wide QRS tachycardia in a young patient strongly suggests VT; however, other possibilities include (Fig. 13.5):
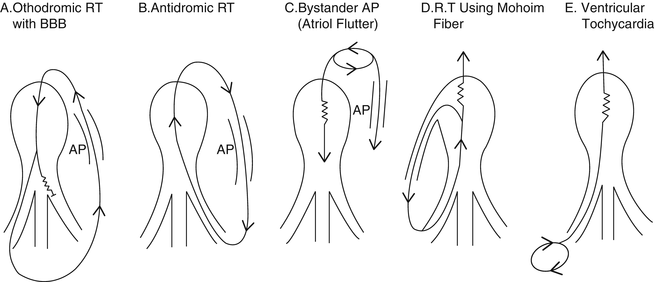

Fig. 13.5
See Differential Diagnosis [Reprinted from Benson DW, et al. Mechanisms of regular wide QRS tachycardia in infants and children. American Journal of Cardiology 1982;49(7):1778–1788. With permission from Elsevier.]
Supraventricular arrhythmia with rate-related aberrancy (Figs. 13.5a and 13.6)
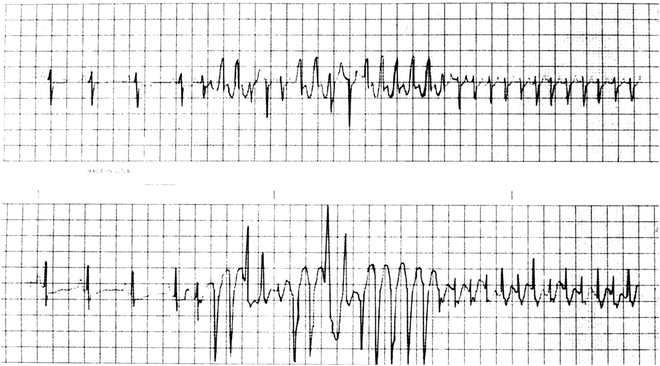
Fig. 13.6
Holter tracing of an infant with SVT and aberrancy [Figure is not a continuous ECG]—i.e., rate-related bundle branch block—both right (positive-wide QRS complexes) and left (negative-wide QRS complexes)—due to the atrial tachycardia impulses encountering relative refractoriness in the bundle branches. The refractory periods shorten in response to the shortened cycle lengths of the tachycardia, eventually normalizing the QRS complex (right-sided beats of upper and lower tracing)
Antidromic supraventricular tachycardia (Fig. 13.5b)
An atrial tachycardia with preexcitation, including a Mahaim fiber (Fig. 13.5c, d)
Ventricular tachycardia (Fig. 13.5e)
Tachycardia with a preexisting bundle branch block
A ventricular paced rhythm
Aberrant conduction (aberrancy) results from a delay in the recovery of the relative refractory period of the bundle branches (usually the right as it has a longer refractory period than the left) as the heart rate changes quickly producing a left or right bundle branch morphology (Figs. 13.5a and 13.6).
At times preexisting wide QRS morphology may manifest as an abnormal wide QRS tachycardia driven by an atrial tachycardia (Fig. 13.7). When possible, especially in patients with congenital heart disease, comparison of the tachycardia QRS morphology to a baseline ECG may be helpful in distinguishing a preexisting wide QRS from a true ventricular arrhythmia.
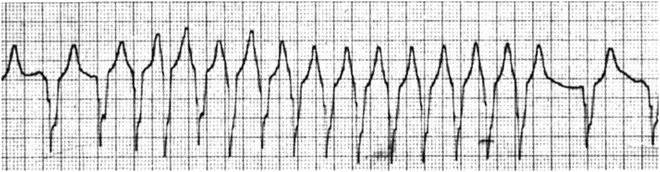

Fig. 13.7
Wide QRS tachycardia in a patient with transposition of the great arteries and ventricular septal defect following the Rastelli operation. Note the first and the last two slow wide QRS complexes are identical to the wide QRS complex tachyarrhythmia, indicating that the wide QRS complex tachycardia is due to a preexisting conduction abnormality
Other forms of wide QRS tachycardia include antegrade conduction during both sinus rhythm and SVT through a Mahaim fiber (Fig. 13.5d) or through an atriofascicular accessory pathway (not shown in Fig. 13.5); both of these anomalous connections are right sided so the wide QRS morphology in both sinus rhythm and SVT is similar and of a LBBB configuration. Rarely, atrial flutter or fibrillation (Fig. 13.5c) in the presence of preexcitation produces a wide complex rhythm due to rapid conduction through the accessory pathway. In other unusual cases, an accessory pathway may provide the atrioventricular limb of a reentrant circuit maximally activating (preexciting) the ventricular myocardium followed by retrograde (antidromic) conduction through the His–Purkinje system—AV node to “reenter” the chambers of origin (atria), resulting in an antidromic tachycardia (Fig. 13.5b); (see Chaps. 4 and 20).
Classification of Ventricular Tachycardia
One may classify VT from a variety of frames of reference including duration, morphology, etiology, association with underlying heart disease, relationship to physical activity and presumed origin of the tachycardia of the origin (Tables 13.1 and 13.2).
Table 13.1
Factors to consider when classifying VT
Non-sustained | Sustained |
|---|---|
Monomorphic | Polymorphic |
No heart disease | Structural or function myocardial disease |
Focal myocardial process | Diffuse myocardial process |
Specific drug response (e.g., calcium blocker) | No specific drug response |
Exercise suppressible | Exercise induced |
Automatic or triggered automatic | Reentry or multiple reentrant |
No underlying electrical disease | Underlying electrical disease |
Table 13.2
Classification of VT by duration
Duration of VT | Description |
|---|---|
Salvo | A few beats in a row |
Non-sustained VT | Typically 3–4 beats in a row up to runs of 30s in duration |
Sustained VT | An episode longer then 30s, or requiring termination due to hemodynamic compromise |
Repetitive VT | Multiple salvos or non-sustained VT events in close proximity |
Incessant VT | Lengthy sustained VT that often dominate the cardiac rhythm |
Electrocardiographic Morphology
Monomorphic VT refers to one dominant QRS form during VT whereas polymorphic refers to a changing QRS shape and beat-to-beat interval within the episode.
A form of polymorphic VT is “bidirectional tachycardia” where the QRS alters between one form and another, typically flipping its axis every other beat across the baseline. This may be seen in a portion of patients with catecholaminergic polymorphic VT (CPVT), with digoxin toxicity, or with some inherited arrhythmias. Polymorphic VT is considered more predisposed to degeneration to ventricular fibrillation.
Electrophysiologic Mechanism
Understanding of the mechanism of VT may be important in deciding on either medical management strategies or invasive management strategies. Three known arrhythmia mechanisms occur: reentry, automaticity, and triggered automaticity. Reentrant arrhythmias in pediatric and congenital heart patients are seen in the scar formation after cardiac surgery, with myocardial ischemia due to coronary artery anomalies, or with abnormal coronary perfusion related to chronic abnormal loading conditions. These the scarred areas, by virtue of their slow conduction property and their anatomic relationship to other scarred areas or to naturally occurring electrically inert borders like AV grove, may form corridors of slow electrical conduction through which electrical activity may enter, exit, and then reenter. Stretch on the myocardium may induce an extra depolarization and imitate a reentry VT in patients with abnormal loading condition.
Clinical Presentation
Ventricular tachycardia may present with a variety of clinical scenarios. It is not infrequent to encounter patients who are completely asymptomatic with the diagnosis. Often these young patients have no underlying structural heart disease and may have either repetitive non-sustained VT or sustained but rather slow VT. Those patients with non-sustained VT may be picked up by finding an irregular heartbeat during routine examination. There are some patients who have a sensation of a racing heart others, with minimal symptoms. Associated symptoms during tachycardia may include dizziness, particularly at the onset of tachycardia, vague chest discomfort, some dyspnea, weakness and headache. Surveillance with regular ECG (Holter) monitoring may be helpful; to detect and evaluate this potential late complication.
Sustained VT over time may lead to tachycardia-mediated cardiomyopathy. In the young patient presenting with clinical congestive heart failure in the presence of a chronic, modestly increased rate, VT, in an otherwise structurally normal heart, presents a diagnostic challenge; determination of the primary cause—VT or primary myocardial disease—is required. VT secondary to a primary dilated cardiomyopathy may be best treated with medication and possibly implantable cardio-defibrillator, though the value of the defibrillator is unproven. On the other hand, tachycardia induced heart failure patients may best benefit from primary treatment of the tachycardia either through medication or catheter ablation. Often the cardiac function will return to normal with the elimination of symptoms.
The most serious but rare clinical presentation of VT in the young is syncope or sudden death. These presentations are unusual in a structurally normal heart save for those with underlying electrical disease (inheritable arrhythmia syndromes, see Chap. 19) or those with infiltrative or hypertrophic diseases of the myocardium. Rather, the number of patients with this presentation will be those who have had surgery for congenital heart disease or those who have an acutely or chronically dysfunctional myocardium after infectious or inflammatory insult.
Clinical Investigation
History
As seen in patients with supraventricular tachycardia, patients who perceive VT will typically report a sudden onset and cessation of the arrhythmia. A history of acquired or congenital heart disease and therapy, substance abuse, possible exposure to toxins or contact with infectious agents (viruses) is important. The family history should be explored vigorously, often with a trained genetic counselor, focus on syncope, seizures, sudden death, or specific inheritable cardiac arrhythmia diagnoses.
The Physical Exam
During an arrhythmia, the examination findings reflect the presence of the tachycardia as well as the inefficiency of cardiac contraction and the AV dissociation. The rate would be elevated and pulses may be weak. The observation of cannon waves in the neck reflecting episodic atrial contraction against a closed AV valve would be useful. Diastolic filling sounds may reflect underlying primary cardiomyopathic condition. Abnormal heart sounds, murmurs, rubs, and chest scars will suggest the likely presence of congenital heart disease and the related surgical palliations or corrections. Rarely will VT will respond to vagal maneuvers.
Echocardiogram
Patients with ventricular arrhythmias require an echocardiogram to rule out structural heart disease and to evaluate cardiac function. In addition for those without congenital heart disease, echocardiography (with Doppler) should focus on possible tumors, ventricular hypertrophy as well as myocardial dysfunction brought on by abnormalities of preload or afterload or by conditions primarily affecting contractility.
Cardiac Magnetic Resonance (CMR)
MRI appears to offer the advantage of viewing the ventricles in three dimensions and it may give a better view of both anatomy and function. In addition, it provides a useful sensitivity for detecting myocardial abnormalities such as areas of fatty replacement in the patient with arrhythmogenic right ventricular dysplasia. Delayed enhancement and the presence of fibrosis on CMR have also been suggested as a marker of risk for a patient to develop arrhythmias in hypertrophic cardiomyopathy and other congenital heart populations. In selected cases with scar-related VT, CMR may also assist in the planning process for scar/fibrosis mapping in patients who may undergo an electrophysiologic study and ablation. In selected cases, CMR images may be imported into and merged with electro-anatomical mapping systems used during catheter electrophysiologic assessment to enhance and clarify points of intracardiac anatomy.
Holter Monitor
Holter monitoring is useful for assessing the results of ablation or medications. It can also give one a measure of the density (percentage of tachycardia beats relative to total beats) of non-sustained VT episodes that may serve to trigger sustained VT episodes.
Treadmill Exercise Testing
Treadmill exercise testing can be helpful for diagnosis of exercised-provoked VT (such as CVPT) and in ascertaining the effectiveness of antiarrhythmic therapy or ablative therapy. Some tachycardias are suppressed by exercise and increasing sinus rates but the prognostic significance of that observation is unclear.
Hemodynamic Catheterization
As part of the electrophysiologic study, hemodynamic data and angiography may be helpful to determine the current hemodynamic status of the heart and circulation, especially in patients with congenital heart disease. Since the primary goal in the management of ventricular tachyarrhythmias in this population is to first improve the hemodynamic status with medical or surgical therapy when appropriate, hemodynamic catheterization and angiography can provide directions. In addition, the coronary arteries are best imaged by the transcatheter-selective coronary approach.
Electrophysiology Study With/Without Ablation
Electrophysiologic investigation may be indicated for those patients with documented non-sustained VT, symptoms suspicious for tachyarrhythmia or a high density of premature ventricular systoles. It may also be indicated to evaluate for sustained VT in those patients with underlying structural heart disease and the effectiveness of orally administered antiarrhythmic therapy. The goal is to determine if the patient has inducible VT that places the individual at higher risk. Even so, sensitivity and specificity of an EP study remains imperfect for prognosis of an arrhythmia.
An ablation may be considered in patients presenting VT if there is substrate that may be effectively eliminated. This is often considered the first line for patients with a monomorphic VT associated with symptoms or functional/structural abnormalities. In others, without a substrate that can be reliably ablated such as channelopathies, or polymorphic VT, an ICD should be considered in place of ablation therapy.
Common Types of Ventricular Tachycardia in the Pediatric Population
Accelerated Ventricular Rhythm (AVR)
This arrhythmia simulates VT but is usually not considered a VT. It has characteristics similar to VT (Figs. 13.8 and 13.9).
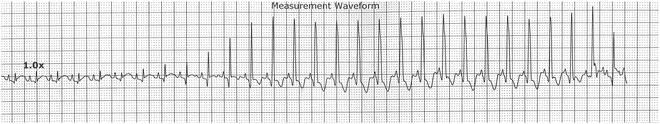

Fig. 13.8
Accelerated ventricular rhythm in 22-day-old infant at virtually the same rate as the sinus tachycardias. Note the fusion beats at the beginning of the AVR and at the end as the sinus impulse partially captures ventricular activation. Note also the more narrow QRS of the AVR that is likely age related

Fig. 13.9
Ten-year-old girl with a normal structural heart and a “slow” ventricular rhythm
Diagnostic ECG Features of AVR
(a)
At least three beats of a ventricular rhythm which is ≤125 % of the rate of the sinus rhythm.
(b)
Fusion beats often appear at the onset and termination of the arrhythmia.
Background
AVR is seen in all age groups and has been described in the presence of heart disease. In pediatrics, it segregates to the infant age group and is usually found in patients with a normal heart while in older adults it may be associated with coronary artery disease. It has also been seen with medications, digitalis intoxication, myocarditis, and other cardiomyopathies.
< div class='tao-gold-member'>
Only gold members can continue reading. Log In or Register to continue
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


