Fig. 23.1
Transseptal puncture access to left atrium: LAO fluoroscopy view during process of transseptal puncture. Decapolar catheter in coronary sinus. Quadripolar catheter in high right atrium. His/RV combo catheter obscured in a perpendicular to the image position. Panel (a) shows the transseptal apparatus positioned on the septum. Contrast has been injected into the septum creating a stain. The transseptal needle is protruding through the septum into the left atrium and the dilator is tenting the septum. In panel (b) the dilator has been advanced over the needle into the left atrium. The sheath is being advanced over the dilator and is crossing the septum marked by the stain. In panel (c) the sheath has been positioned across the septum and a radiofrequency ablation catheter is advanced through it to the lateral mitral valve annulus
In small children, the anterior–posterior dimension of the LA is particularly shallow, so avoidance of entering the posterior pericardial space is critical. Procedural modifications for failure of “first pass” transseptal puncture include removal of the needle and rewiring the sheath/dilator in the SVC to begin the procedure again, manual formation of modified curvature on the needle, or changing to a sharper needle type. Procedural modifications for patients with congenital heart disease include different needle orientation to adjust for variations in postoperative intracardiac anatomy. For lateral tunnel or extra-cardiac Fontan anatomy, the needle orientation remains leftward but slightly more anterior (2 o’clock position). Patients with Mustard or Senning intra-atrial baffle, the needle is typically oriented rightward and anterior (“11 o’clock”). Pre-procedural imaging such as CT or MRI can be very useful in planning the approach for transseptal or trans-baffle access in congenital heart disease patients.
Because the sheath, catheter, and delivery of RF on the left side of the heart present a risk of thrombus formation, anticoagulation with heparin is delivered during the procedure once entry into the LA is achieved. Bolus and/or continuous heparin infusion is delivered to maintain an activated clotting time of at least 220 s. If RF is delivered to the left side of the heart, the patient is placed on 81 mg of aspirin a day for 6 weeks following the procedure.
Catheter Navigation and Mapping
In the past, fluoroscopy has been the primary method by which catheter position was determined. The development and adaption of computerized electroanatomic mapping has revolutionized catheter navigation in cardiac electrophysiology. Commonly used systems are Ensite (St. Jude Inc., St. Paul, MN) and CARTO (Biosense Webster, Diamond Bar, CA). Both systems allow for “virtual” visualization of the ablation catheters movement within the space of the heart. This allows for catheter manipulation without the use of fluoroscopy. The Ensite system uses a localization system based on the changes in electrical impedance across the chest and can “visualize” (display on a monitor screen) any electrophysiologic catheter within the heart. CARTO systems utilize changes in the magnetic fields across the chest to “visualize” the ablation catheter; the newer iteration allows for “visualization” of all diagnostic catheters as well.
Critical to the ablation procedure is mapping the arrhythmia substrate—focus or reentrant pathway—to identify the target for ablation. There are several methods by which to map and target the substrate depending on the arrhythmia diagnosis. Anatomic targeting is the primary method for the slow pathway in AVNRT. A component of anatomic mapping is also essential for accessory pathway ablation, as the catheter must target the annulus between the atrium and ventricle. Pace mapping is a technique used in mapping ventricular arrhythmia substrates. In this technique, the ablation catheter is used to pace the heart at various locations in order to create a paced QRS morphology which matches the arrhythmia QRS morphology—suggesting the site is the origin of the arrhythmia breakout. Entrainment mapping is the process of identifying the course of a macroreentry tachycardia circuit using entrainment techniques (Chap. 3). This method can be used for intra-atrial reentry and ventricular reentry circuits. Voltage mapping can identify areas of scar or low voltage “bridges” in the heart which may be the substrate leading to reentry circuits. By far, the method most used is activation mapping, by which the timing of the local electrical activation is utilized to identify the location of the substrate. This may be the earliest electrical activity at a specific focus or at the earliest atrial or ventricular activation at the site of an accessory pathway.
Computerized electroanatomic mapping systems are used primarily for activation mapping but are also used for voltage mapping. These systems create a visual representation of the endocardial chamber based on local data points generated as the mapping catheter is moved within the chamber of interest. Local activation time is measured at any point the catheter contacts and compared to a predetermined reference location. The system then color coordinates the activation times, such that the earlier activated areas are differentiated from subsequently activated areas. Focal arrhythmia substrates essentially show a “bull’s-eye” pattern with a single site being the earliest (Fig. 23.2). Macroreentrant arrhythmias will display a circumferential pattern with the latest activation sites continuous with the earliest activation sites (Fig. 23.3).
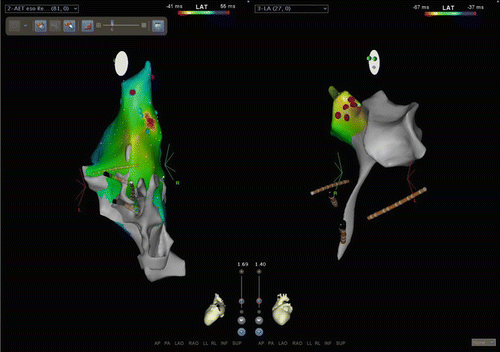
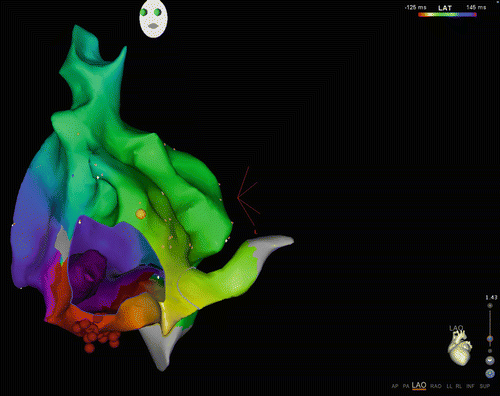

Fig. 23.2
CARTO3 screenshot of focal atrial tachycardia ablation. Left panel is a left lateral (slight posterior) view of the right atrium. Activation map of a focal atrial tachycardia is displayed. Red is the earliest sight with timing progressing through the spectrum to blue. This local area of activation was the target for ablation. RF here was not successful. This location in the RA was immediately anterior to the right upper pulmonary vein. The right panel is a left lateral view (slight anterior) of the left atrium (the “tail” inferiorly is the tract through the transseptal access point). The red dots on the map indicate the area targeted for ablation which coordinated with the point of interest on the RA map

Fig. 23.3
CARTO3 map of macroreentry tachycardia: The view is LAO from the apex as demonstrated by the heart diagram in the right lower corner. The tricuspid valve annulus is shown as a “hole” in the map, giving the impression of looking “inside” beyond the valve (“M shaped shadow” in lower part of figure). The yellow ball in the right upper aspect of the tricuspid valve annulus is the location of the His. This is a map of CTI-dependent (typical counterclockwise) atrial flutter. The color pattern from red to green to blue reflects timing as the wave front moves counterclockwise around the tricuspid valve annulus. The late site meets the early site of activation at the red strip (purple meets red) indicating a continuous reentry circuit. The red dots at the inferior portion of the map are radiofrequency ablation lesions placed to terminate the tachycardia
Both CARTO and Ensite use contact mapping, in which the catheter must be in contact with the endocardium to register a local electrogram. The Ensite system also allows for non-contact mapping—this currently requires a multi-electrode balloon catheter be placed in the chamber of interest; the electrogram timing is calculated from the far-field unipolar electrograms detected by the electrodes on the balloon surface. The benefit of non-contact mapping is that the entire arrhythmia circuit can be mapped in a single beat. The negative aspect of the non-contact mapping system is that the balloon catheter is rather large profile requiring a large sheath for placement and cannot readily be used in a smaller child.
Additional benefits to these mapping systems are the ability to incorporate additional images into the system. Previously obtained MRI or CT images of the cardiac anatomy can be uploaded into either Ensite or CARTO systems and used as the cardiac geometry for mapping the arrhythmia. CARTO also can incorporate intracardiac ultrasound imaging to create a detailed intracardiac anatomy.
Approach to Accessory Pathways
Basic terminology for describing accessory pathways is found in Table 23.1. The nomenclature for accessory pathway locations has been in a slowly evolving process over the past 2 decades. Initially the valve annuli have been described from the en face view of the valve from the RV apex and comprised anterior, posterior, septal, and lateral segments, with anterior being the portion near the aortic root and AV node and posterior being near the CS and CS os. The origin of this nomenclature is from early pathologic and surgical descriptions with the heart oriented in a non-anatomical position. In 1999, a NASPE (now HRS) consensus statement was published to “correct” this terminology to superior (anterior), inferior (posterior), septal, posterior (mitral valve lateral), and anterior (tricuspid valve lateral). Adaption of this terminology has been slow, especially in the pediatric literature. The new terminology of superior and inferior is anatomically accurate and easy to interchange with the former anterior and posterior terminology, and has been widely adopted in many publications. There is obviously potential confusion with re-using the terms anterior and posterior to define the lateral annuli and this change has not been adopted. The descriptions of the annuli in this chapter will support the new terminology, using superior, inferior, and septal; but retain the use of left lateral and right lateral to describe the annular free walls (Fig. 23.4).
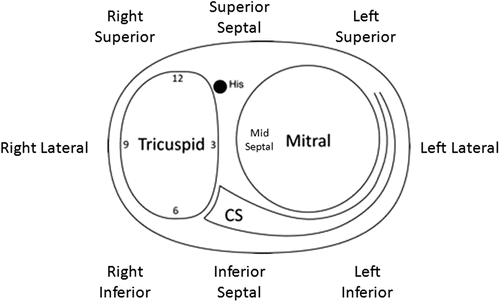
Table 23.1
Terminology used in describing accessory pathways (APs)
Orthodromic | Describes AP mediated tachycardias in which there is normal conduction from the atria to the ventricle via the AV node-HPS |
Antidromic | Describes AP mediated tachycardias which traverse the AP from atrium to ventricle and proceed backwards up the HPS |
Antegrade | Conduction from atria to ventricle |
Retrograde | Conduction from ventricle to atrium |
Concealed | Pathway only conduct in the retrograde direction |
Manifest | Pathways conduct in the antegrade direction with or without retrograde conduction |

Fig. 23.4
Annular nomenclature for accessory pathway locations: cartoon of the annulus as viewed from the apex in the left anterior oblique view. The “new” annular anatomy is labeled. Correlated to traditional terminology: superior = anterior; inferior = posterior. CS coronary sinus
For both left- and right-sided accessory pathways, the amplitude of the atrial or ventricular electrograms confirms optimal proximity to the AV ring. Due to the dominant ventricular mass relative to the atrium, the ratio of the amplitude of the atrium to that of the ventricle should usually be less than 0.5. At the beginning of the ablation era, much of the inference that is contained in the amplitude of the electrograms and in the VA conduction times was dependent on operator integration of the anatomic and electrical data on the fly; more recently, computer-assisted mapping techniques produce an electro-anatomic map correlating in a three-dimensional image the activation sequence and the anatomic configuration.
Left-Sided Pathways
Approximately 60–65 % of accessory pathways are located on the left side. This left-sided dominance is higher in infants and small children. As the infant or child grows, functional or anatomic loss of the pathway may occur, perhaps due to molding of the AV groove or postnatal apoptosis of the cellular pathway substrate. Such a natural attrition would reduce the absolute incidence of left-sided pathways. In contrast, right-sided pathways may theoretically be somewhat protected from this “molding effect” by their closer proximity to the crux and by the fixation of the right side by the vena cavae compared to the relatively less tethered left lateral aspect of the heart.
Access to the left side is by way of the transseptal technique into the LA. Once in the LA, there should be free movement of the curve and catheter tip so that it can sweep in an arch from near the top of the LA down to the mitral inferior (posterior) rim for mapping and ablation. Left inferior (posterior) pathways require a slightly tighter curl to the catheter curve, achieved by gently decreasing the radius of the curve and moving the curved tip inferiorly by slightly withdrawing the catheter and sheath, and thus positioning the electrode–ablation tip slightly more medial on the inferior (posterior) mitral ring. Further tightening the curve and further withdrawing slightly the catheter and sheath will bring the catheter tip to the left inferior (posterior) septal region. The mapping and ablation apparatus (catheter and sheath) require continued clockwise torque on the catheter–sheath apparatus to ensure the catheter remains on the inferior (posterior) mitral ring. The superior septal region, the last site on the mitral ring, is a particularly difficult location to secure a stable position of the catheter tip, though this is adjacent to the aorto-mitral continuity and APs in this location are extremely rare. Long sheaths specifically formed to position the catheter on the mitral annulus (and even steerable sheaths) are available and commonly used to position the catheter and provide for greater stability. Delivery of radiofrequency energy with the catheter tip at the mapped site of the accessory pathway often will ablate the pathway and terminate the tachycardia.
Left-sided accessory pathways are targeted by activation mapping. Several approaches may be used. The first is to map the site of earliest local preexcitation (Fig. 23.5) in patients with manifest pathways during sinus rhythm or an atrial pacing; local preexcitation at an optimal ablation site is usually ≥15 before the onset of the surface QRS. The second is to locate and the accessory pathway by mapping the retrograde activation sequence during ventricular pacing (Fig. 23.6) in patients with either manifest or concealed pathways. One must pace at a sufficiently fast rate (usually 150 bpm or greater) to ensure that retrograde conduction through the accessory pathway is not masked by robust retrograde AV nodal–His-Purkinje conduction often seen in young patients. Mapping may be performed during tachycardia; however, because abrupt termination can displace the catheter tip, ventricular pacing slightly faster than the tachycardia rate should be initiated prior to applying RF. Energy is then delivered during ventricular pacing which is continued for the duration of the lesion (Fig. 23.6). Cryoablation also provides secure catheter stability due to catheter adhesion. However cryoablation is not extensively used for this purpose due to a perceived higher recurrence risk.
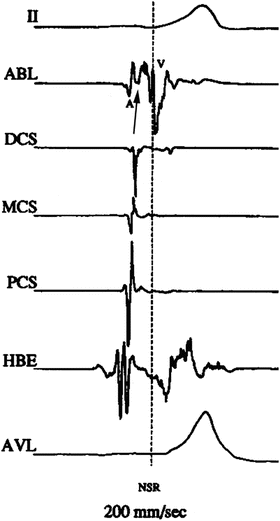
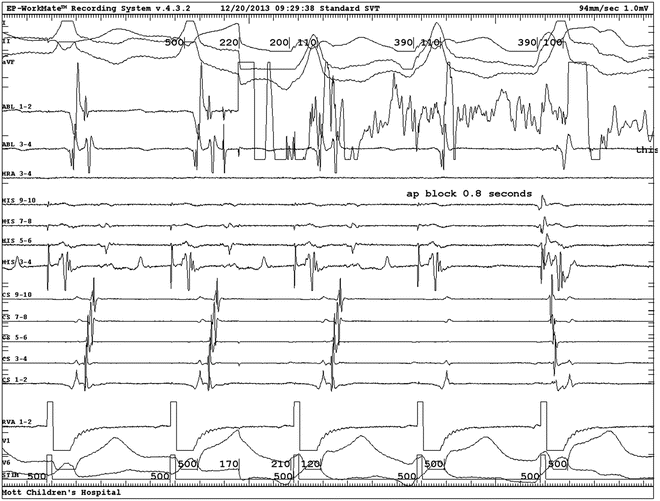

Fig. 23.5
Activation mapping of preexcitation: intracardiac electrograms displayed from top to bottom: surface lead II, ablation distal, distal CS, mid-CS, proximal CS, His bundle, surface lead aVL. Single beat with ventricular preexcitation. No His potential because it is buried in the ventricular electrogram. Note the local ventricular preexcitation in the ablation electrogram (arrow) precedes the global preexcitation expressed in the two surface leads. A atrial electrogram; V ventricular electrogram

Fig. 23.6
Activation mapping and ablation of left lateral accessory pathway: intracardiac electrograms displayed from top to bottom: surface leads I, II, aVF, ablation distal to proximal, HRA, His proximal (9-10) to distal (3-4), CS proximal (9-10) to distal (1-2), RV apex, surface leads V1 and V6. Right ventricular pacing conducts to the atrium via a left lateral accessory pathway with CS activation proceeding from distal to proximal. The earliest activation is at the distal ablation electrogram. Application of radiofrequency energy causes artifact on the distal ablation electrogram and results in accessory pathway block at the fourth beat of the display. HRA high rate atrium, CS coronary sinus, RV right ventricle
Retrograde conduction through left-sided concealed pathways may be difficult to isolate if retrograde AV nodal conduction is vigorous. Two solutions are available. The first is the bolus administration of adenosine. This technique will usually block the AV node retrograde, but usually not the accessory pathway. However, its transient effect limits it usefulness. The second solution is to pace the ventricle from a site closer to the accessory pathway. This may usually be achieved with pacing from the septal base in the right ventricle. Occasionally, an LV pacing site is necessary and can be achieved by a second catheter places across the atrial septum, a retrograde catheter placed from the arterial side, or a CS catheter positioned down a lateral cardiac vein. This technique more selectively engages the left accessory pathway than does pacing from the RV apex, thus unmasking conduction in the left-sided pathway (Fig. 23.7).
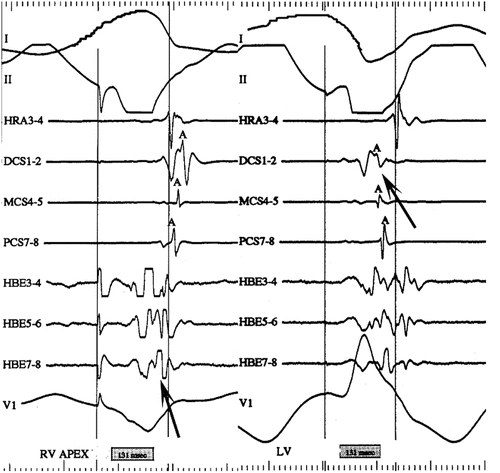

Fig. 23.7
LV pacing to isolate left lateral accessory pathway conduction: intracardiac electrograms displayed as surface lead I, II, HRA, distal CS (DCS), mid-CS (MCS), proximal CS (PCS), His bundle (HBE) distal to proximal, surface lead V1. Left-hand panel: pacing from the RV apex activates the atrium retrograde with earliest activation in at the His catheter. Right-hand panel: pacing from the LV posterior wall activates the atrium retrograde via a left lateral accessory pathway earliest at the distal CS. HRA high right atrium, CS coronary sinus, RV right ventricle (HRA), MCS mid-coronary sinus, PCS proximal coronary sinus
Right-Sided Pathways
Access to right-sided structures is clearly less complicated than access to the left. On the other hand, catheter stability on the AV ring, especially along the right free wall and superior (anterior) areas, is less secure. In addition, delivery of radiofrequency energy to the tricuspid AV ring in the septal area places the AV node, His-Purkinje system, and AV nodal artery at greater risk.
Right-sided accessory pathways are targeted by activation mapping similar to left-sided accessory pathways. Earliest local preexcitation, usually ≥15 ms before the onset of the surface QRS, may be targeted during sinus rhythm if the pathway is manifest. Retrograde activation may also be targeted during ventricular pacing in patients with either manifest or concealed pathways. Isolation of right-sided APs during ventricular pacing is typically straightforward due to the RV catheter’s proximity to the AP, though superior (anterior) and mid-septal APs may be very difficult to differentiate from AV nodal conduction. Mapping may be performed during tachycardia, however because abrupt termination can displace the catheter tip, ventricular pacing slightly faster than the tachycardia rate should be initiated prior to applying RF. Energy is then delivered during ventricular pacing which is continued for the duration of the lesion.
< div class='tao-gold-member'>
Only gold members can continue reading. Log In or Register to continue
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


