Ventricular Tachycardia
David Wilber
Overview
Ventricular tachycardia (VT) remains an important cause of morbidity and mortality in cardiac patients. Symptoms may be mild (palpitations and dyspnea), or they may reflect rapid and severe hemodynamic compromise (syncope, cardiac arrest). In occasional patients, congestive heart failure may be the initial presentation when VT of prolonged duration remains unrecognized. Structural heart disease (SHD) is present in 85% to 90% of patients, with healed myocardial infarction the most common cause. The specific underlying myocardial substrate has an important influence on long-term outcome and therapeutic options, and it is discussed in detail in subsequent sections.
Although rapid rates (>200 beats per minute) are more likely to produce hemodynamic compromise (1,2,3), autonomic compensatory mechanisms (particularly the baroreceptor reflex) play a major role independent of heart rate or pre-VT hemodynamic status (4,5). Impairment of this reflex has been associated with more rapid hemodynamic deterioration (5). Unstable VT is an important, but by no means exclusive, cause of cardiac arrest and sudden death. The exact proportion of cases in which VT is the initiating rhythm is difficult to ascertain, because it may transition rapidly to ventricular fibrillation (VF) or asystole and is thus underestimated by rhythms recorded by “first responders” at the time of arrest. Similarly, data from stored electrograms of implantable cardioverter-defibrillator (ICD) recipients may not accurately reflect rhythms at the onset of cardiac arrest, because many ICD-treated episodes may have self-terminated or been sufficiently stable to permit survival even without immediate intervention (6).
Increasing evidence indicates that the degree of hemodynamic stability at initial clinical presentation has little impact on long-term prognosis in patients with SHD. In the Antiarrhythmic vs Implantable Defibrillator (AVID) Registry (7), the mean ejection fraction was slightly higher in 440 patients presenting with stable VT (34% ± ∀13%) than in 1,029 patients presenting with unstable VT (31% ± 11%); congestive heart failure was also less common in patients with stable VT (34% versus 45%). Prior myocardial infarction, present in 72% of patients, and other cardiac diagnoses were similar between the two groups. Overall, increasing age, lower ejection fraction, congestive heart failure, nonuse of β-blockers, and ICDs were significant multivariate predictors of mortality. Hemodynamic stability of VT at presentation was not a predictor of mortality in univariate or multivariate analysis. These findings are corroborated by the frequent occurrence of rapid unstable VT during long-term follow-up in patients presenting with stable VT treated by an ICD (8,9).
General Aspects of Diagnosis and Management
Electrocardiographic Recognition
VT originates from ventricular muscle, specialized conduction tissue (bundle branches and Purkinje fibers), or elements of
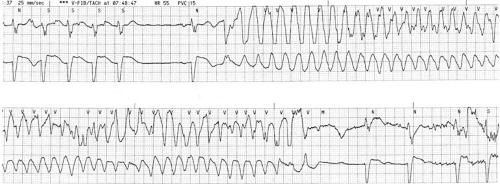
both. Although reentry is the most common mechanism, both normal and abnormal automaticity and triggered activity may play a role (see Chapter 57). Identification of VT begins with the recognition of three or more consecutive wide (≥120 milliseconds) complexes. Rarely, the QRS complex may be slightly narrower if the focus is within or adjacent to the proximal Purkinje system or bundle branches, with rapid early engagement of conduction over this network (10). These complexes may have a uniform appearance from beat to beat (monomorphic VT), or consecutive complexes may vary, often widely, in QRS configuration (polymorphic VT) (Fig. 66.1). A specific pattern of polymorphic VT, termed torsade de pointes, manifests a periodic reversal of QRS polarity associated with waxing and waning QRS amplitude. The distinction between monomorphic and polymorphic VT has important implications in terms of mechanism, underlying substrate, and prognosis that are discussed in subsequent sections. Spontaneous termination within 30 seconds is generally designated nonsustained VT, with longer durations considered sustained. Very rapid rates (>270 beats per minute) are seldom associated with discrete identifiable QRS complexes and are usually designated VF.
both. Although reentry is the most common mechanism, both normal and abnormal automaticity and triggered activity may play a role (see Chapter 57). Identification of VT begins with the recognition of three or more consecutive wide (≥120 milliseconds) complexes. Rarely, the QRS complex may be slightly narrower if the focus is within or adjacent to the proximal Purkinje system or bundle branches, with rapid early engagement of conduction over this network (10). These complexes may have a uniform appearance from beat to beat (monomorphic VT), or consecutive complexes may vary, often widely, in QRS configuration (polymorphic VT) (Fig. 66.1). A specific pattern of polymorphic VT, termed torsade de pointes, manifests a periodic reversal of QRS polarity associated with waxing and waning QRS amplitude. The distinction between monomorphic and polymorphic VT has important implications in terms of mechanism, underlying substrate, and prognosis that are discussed in subsequent sections. Spontaneous termination within 30 seconds is generally designated nonsustained VT, with longer durations considered sustained. Very rapid rates (>270 beats per minute) are seldom associated with discrete identifiable QRS complexes and are usually designated VF.
Most wide complex tachycardias (∼80%) are ventricular in origin, particularly in the presence of known SHD, and this prior probability should influence subsequent decision making (11,12). Supraventricular tachycardia (SVT) may be associated with a wide QRS complex resulting from (a) preexisting intraventricular conduction defects, (b) aberrant conduction (from incomplete repolarization of some portion of the His-Purkinje system during tachycardia), (c) conduction over an accessory pathway, or (d) conditions associated with depressed conduction (drugs, metabolic or electrolyte abnormalities, ischemia). Rarely, ventricular pacing at rapid rates may cause diagnostic confusion because of failure to recognize the often imperceptibly small pacing artifacts on the surface electrocardiogram (ECG).
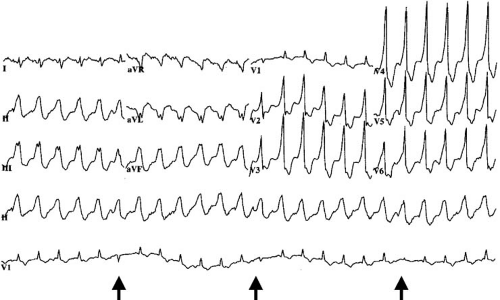
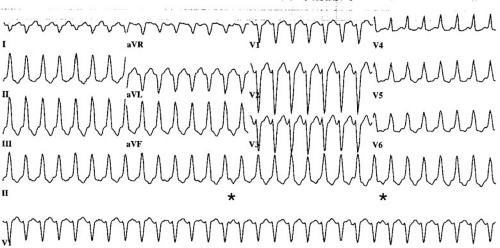
Distinction between VT and SVT with aberration can be difficult in individual patients, but several general principles are useful (Table 66.1). Capture beats and fusion beats are generally diagnostic for VT, but they are present in only a small number of cases (Fig. 66.2). Rarely, a ventricular ectopic beat during aberrantly conducted SVT can incorrectly suggest VT. Atrioventricular dissociation indicates VT with rare exceptions (Fig. 66.3). It is present in up to 70% of VTs and is more common at rapid rates. It also can be suspected if cannon A waves are observed during inspection of the jugular venous pulse during physical examination. The wider the QRS duration, the more likely the rhythm is VT. Durations of 160 milliseconds or longer during left bundle branch block patterns and of 140 milliseconds or longer during right bundle branch block patterns are useful guidelines (10). A frontal plane axis between –90 and + 180 degrees (“northwest axis”) strongly favors VT. The precordial R/S criterion originally proposed by Brugada is relatively specific for VT (13). The criterion is present if either no RS complexes occur in the precordial leads, or, if R/S complexes are present, the interval from onset of the R wave to the nadir of the S wave is greater than 100 milliseconds. The absence of a typical bundle branch block pattern or rapid precordial intrinsicoid deflection favors VT. In the setting of preexisting intraventricular conduction defects, differences in QRS morphology between the baseline ECG and that of tachycardia favor a diagnosis of VT; however, similarity between the two does not exclude VT. Although numerous additional criteria have been proposed based on QRS configuration in specific leads, most have relatively low positive predictive value or are applicable only if the baseline QRS complex is normal.
Acute Therapy
Tachycardia associated with significant hypotension, heart failure, or angina should be promptly terminated by direct current cardioversion. In patients with recurring or incessant VT, evidence indicates that intravenous amiodarone leads to rapid arrhythmia control and may be superior to other antiarrhythmic therapy (14,15,16). In the setting of acute myocardial ischemia and unstable VT, lidocaine has potential benefits in preventing recurrences (17), although there are limited data to suggest efficacy in this setting. β-Blockers, administered intravenously if needed, are effective in treating the heightened sympathetic tone that often accompanies episodes of electrical storm (18,19). For similar reasons, sedation and occasionally general anesthesia are also useful. Angiography should be considered early in the course of management to assess the potential for ongoing myocardial ischemia. Other reversible factors, such as electrolyte abnormalities, proarrhythmic medications (including exogenous β-agonists), and hypotension should be identified and corrected. Intraaortic balloon counterpulsation may improve myocardial perfusion and hemodynamics and may ameliorate the consequences of recurring episodes (20). Rarely, ventricular assist devices and cardiac transplantation are required when other measures fail.
TABLE 66.1 Electrocardiographic Criteria for the Diagnosis of Ventricular Tachycardia | ||
|---|---|---|
|
In patients with hemodynamically stable monomorphic VT, the reported efficacy of lidocaine for VT termination varies between 8% and 25% (21,22,23), although this drug continues to
be used because of safety and simplicity of administration. Intravenous procainamide terminates the majority of stable VTs (23), and it may result in more rapid termination in this setting. Placement of a temporary transvenous pacemaker for pace termination of VT is a useful alternative, with the advantage of allowing rapid treatment of recurring episodes. In contrast, constant pacing at modestly fast rates is rarely effective as a preventive measure for monomorphic VT. Polymorphic VTs (e.g., as seen in congenital or acquired long QT) are more likely to be pause or bradycardia dependent. In these patients, institution of pacing at physiologic rates (70 to 100 beats per minute) may dramatically reduce VT frequency.
be used because of safety and simplicity of administration. Intravenous procainamide terminates the majority of stable VTs (23), and it may result in more rapid termination in this setting. Placement of a temporary transvenous pacemaker for pace termination of VT is a useful alternative, with the advantage of allowing rapid treatment of recurring episodes. In contrast, constant pacing at modestly fast rates is rarely effective as a preventive measure for monomorphic VT. Polymorphic VTs (e.g., as seen in congenital or acquired long QT) are more likely to be pause or bradycardia dependent. In these patients, institution of pacing at physiologic rates (70 to 100 beats per minute) may dramatically reduce VT frequency.
Long-Term Management
Once the diagnosis of VT is established or strongly suspected, it is important to develop a detailed understanding of the underlying anatomic and physiologic substrate. This evaluation includes objective assessment of left ventricular function, evaluation of coronary artery patency, and the search for evidence of reversible myocardial ischemia. Other diagnostic testing to establish the presence and nature of underlying SHD may be appropriate in individual circumstances, as discussed in subsequent sections. Electrophysiologic testing (see Chapter 61) has played a diminishing role in the evaluation and management of patients presenting with sustained VT. It is at best a modest predictor of recurrent spontaneous VT and is of little value in the selection of antiarrhythmic therapy. Its primary applications for VT are as a means to establish a diagnosis in wide complex tachycardia of uncertain origin and as part of a potential or planned catheter ablation procedure.
The primary goal of therapy is to alleviate symptoms, to minimize or prevent recurrent VT, and to enhance long-term survival. Data from multiple clinical trials, discussed in detail in Chapter 67, indicate a significant survival benefit for ICD therapy in most patients presenting with spontaneous sustained VT and SHD, with the greatest benefit in patients with severely reduced ventricular function (24). In patients without SHD, limited data support the benefit of ICD therapy in many patients presenting with polymorphic VT and syncope, but there appear to be few ICD indications for those presenting with monomorphic VT (discussed later). However, ICD recipients with SHD remain at high risk of death over subsequent years, with a 3-year mortality of 20% to 25% (24). In patients with severe left ventricular dysfunction and prior myocardial infarction, accumulating data indicate that clinical presentation with sustained VT identifies a group of patients at higher risk for subsequent decompensated heart failure and nonarrhythmic death compared with patients with similar baseline characteristics who never develop sustained VT (25). First onset of sustained VT is often preceded by evidence of clinical deterioration (increased probability of hospitalizations for heart failure and ischemic events) compared with patients who did not develop VT (26). The extent to which current therapy of VT, including the adverse consequences of antiarrhythmic drug therapy or multiple shocks, contributes to this accelerated mortality is unclear at present. However, these data do underscore the importance of a global and comprehensive approach to long-term management, including revascularization, angiotensin-converting enzyme inhibitors, β-blockers (27), and statin therapy (28,29).
Cardiac resynchronization therapy (see Chapter 75) results in long-term improvements in left ventricular size and function, as well as survival. It could be anticipated that such positive remodeling would have a beneficial long-term impact on VT frequency. However, existing data suggest a complex relationship. Investigators have reported no change (30), reductions (30a), or increases (30b) in VT frequency in the months following institution of resynchronization therapy. In the presence of preexisting VT, the direction of this response is likely the result of certain highly patient-specific variables that remain to be elucidated.
TABLE 66.2 Priorities in the Treatment of Frequent Ventricular Tachycardia Episodes | |
|---|---|
|
A large majority of patients with ICDs implanted for spontaneous VT will have therapy delivered during follow-up; in 60% to 70% of patients, “appropriate” therapy will be given for recurrent ventricular arrhythmias (31). These episodes are usually recurrent VT (70% to 80%) and, much less commonly, VF (25,32). In addition, 20% to 25% of patients will have “inappropriate shocks,” most commonly for rapid atrial fibrillation. The incidence of inappropriate shocks has not changed appreciably over the past decade, despite the introduction of dual-chamber devices and enhanced detection algorithms.
For these reasons, additional therapy is often required for ICD recipients (Table 66.2). Antitachycardia pacing (delivery of a programmable number and coupling interval of pacing stimuli at rates faster than the tachycardia cycle length) can terminate VT by prematurely depolarizing a portion of the reentrant circuit ahead of the advancing wavefront. Empirically programmed antitachycardia pacing has been demonstrated to reduce the need for shock therapy substantially, even for relatively fast VTs (cycle lengths, 240 to 320 milliseconds) (33,34,35), and it is associated with improved quality of life (35). Antiarrhythmic drug therapy may improve the response to pacing by slowing the rate and widening the window during which pacing stimuli can penetrate the reentrant circuit. In approximately 20% to 30% of patients, antitachycardia pacing is either incompletely or not effective. There is a small risk of VT acceleration requiring shocks, usually less than 5%, with current pacing protocols.
Several clinical trials have addressed the role of antiarrhythmic drug therapy in preventing first recurrent arrhythmia and ICD therapy following device implantation in patients with SHD who present predominantly with VT. Two class III drugs, sotalol (36,37) and azimilide (38), significantly reduced all-cause shocks, as well as appropriate ICD therapies compared with placebo. In the recently completed Optimal Pharmacologic Therapy in Cardioverter Defibrillator Patients (OPTIC) study, patients were randomized to β-blocker alone, β-blocker plus amiodarone, or sotalol following ICD implantation. The combination of amiodarone and β-blocker was significantly more effective in reducing all-cause shocks and ICD therapies, relative to the other two treatments, although sotalol was marginally more effective that β-blockers alone (39). An important limitation is that both amiodarone and sotalol were withdrawn because of drug intolerance in 20% to 30% of patients during the first 2 years of therapy. For this reason, routine antiarrhythmic therapy in all ICD recipients with VT cannot be recommended, and it should be selectively employed in patients with frequent VT therapies or those requiring suppression of supraventricular arrhythmias. Use of class I antiarrhythmic therapy in patients with SHD should be minimized because of their proarrhythmic potential.
Approximately 10% to 20% of patients with VT will experience at least one episode of “electrical storm,” arbitrarily
defined as three or more ICD therapies within a 24-hour period (40,41,42,43). Acute therapy should follow the guidelines outlined previously. Catheter ablation (discussed in detail in Chapter 73) may be an effective alternative, particularly for patients unresponsive to initial drug therapy or those who are already taking adequate doses of β-blockers and amiodarone. Some (42,43), but not all (40,41), studies indicate that survivors of electrical storm face an accelerated risk of nonarrhythmic death in the months following electrical storm despite resolution of tachycardia. Because few details of therapy were provided in these reports, such differences cannot be reconciled at present.
defined as three or more ICD therapies within a 24-hour period (40,41,42,43). Acute therapy should follow the guidelines outlined previously. Catheter ablation (discussed in detail in Chapter 73) may be an effective alternative, particularly for patients unresponsive to initial drug therapy or those who are already taking adequate doses of β-blockers and amiodarone. Some (42,43), but not all (40,41), studies indicate that survivors of electrical storm face an accelerated risk of nonarrhythmic death in the months following electrical storm despite resolution of tachycardia. Because few details of therapy were provided in these reports, such differences cannot be reconciled at present.
Ventricular Tachycardia Associated with Structural Heart Disease
Coronary Artery Disease
VT commonly arises in the setting of healed myocardial infarction, and it occurs in 1% to 2% of patients during long-term follow-up, often after an interval of several years. Early infarct revascularization has resulted in less aneurysm formation and in potentially smaller scars, but the number of at-risk patients with chronic ischemic cardiomyopathy caused by multiple infarctions and remodeling has increased owing to improvements in long-term medical care (44). The mechanism of VT is usually macroreentry, with focal nonreentrant mechanisms responsible for only 5% to 10% of tachycardias. The reentrant circuits may be several centimeters in length and typically contain at least one region of slowed conduction within the scar (45). The sites of slow conduction during VT consist of surviving muscle bundles with normal action potential characteristics, but reduced cellular coupling resulting from alterations in gap junction number, function, and distribution (46). Slow conduction regions during VT often demonstrate low-amplitude multicomponent delayed potentials detected well after the completion of the surface QRS (47,48). At least some portion of the reentrant circuit is subendocardial in a large majority of patients, but it may be intramural or epicardial as well. Multiple distinct surface ECG morphologies during different episodes of VT are common, reflecting widely separated slow conduction regions or shared areas of slow conduction with variable exits from the scar.
In patients with previous infarction and a history of VT, programmed stimulation during electrophysiologic studies results in induction of VT in 90% to 95% of cases. However, the induced rate and QRS morphology may differ from those observed during spontaneous tachycardia. The link between spontaneous and inducible VT remains incompletely understood. However, the induction of VT signifies the presence of a fixed anatomic substrate associated with an increased likelihood of future spontaneous events (25,49). Triggering factors, including acute ischemia, altered autonomic tone, acute changes in myocardial fiber stretch and wall strain, and metabolic abnormalities, provide the link between susceptibility and spontaneous occurrence of VT. However, the anatomic substrate, once present, may persist indefinitely. Even when VT appears to have transient and reversible causes, long-term risk for recurrent arrhythmias and death continues despite correction of triggering events (50).
Long-term therapy should follow the general guidelines established for patients with SHD and VT outlined previously. Patients with recurrent symptomatic VT despite ICD or drug therapy are candidates for catheter ablation. Conventional mapping techniques target a protected isthmus of slowed conduction as identified by electrogram timing and the response to pacing at various sites during tachycardia (45). This method is limited to the minority of patients in whom a stable tachycardia can be induced. Most patients have multiple stable and unstable tachycardia morphologies, so complete elimination of all VTs can be achieved in only 40% to 50%. However, for many patients this end point is not required; elimination of the clinically problematic or “target” VT is sufficient to reduce the incidence of ICD therapies dramatically and to improve quality of life. This result may be obtained in up to 80% of patients presenting with recurrent stable VT. When performed by experienced clinicians, the procedure is well tolerated, with a 1% to 2% incidence of major complications. With the increasing use of ICDs and catheter ablation, the need for surgical ablation, with its associated higher mortality (10% to 20%), has been largely eliminated. More recently, several investigators demonstrated that critical sites that maintain reentry within the borders of scar can be identified and targeted for ablation during sinus rhythm (51,52,53). This “substrate-based” approach extends the applicability of VT ablation to a much broader range of patients and may improve long-term outcome. Although there is continuing controversy over the need for ICD therapy following acutely successful ablation in all patients with ischemic cardiomyopathy, current consensus favors concomitant use of ICDs in most patients.
Nonischemic Dilated Cardiomyopathy
In patients with nonischemic dilated cardiomyopathy (NDC), subendocardial scarring and patchy fibrosis may occur and may contribute to decreased cellular coupling and slow conduction, thus providing an anatomic substrate for reentrant VT. However, the extent and degree of fragmented and abnormal endocardial electrograms appear to be significantly less than in patients with prior myocardial infarction (54,55). In addition, the distribution of scar is more commonly basal in NDC, contiguous with the mitral annulus. In contrast, epicardial patchy fibrosis, fractionated electrograms, and abnormal conduction may be detected in 30% to 50% of patients with DCM at the time of open heart surgery (56,57,58). Experimental (59) and clinical (58,60) data indicate that focal nonreentrant mechanisms (triggered activity and enhanced automaticity) may be common in patients with NDC. In this population, myocardial macroreentrant circuits may account for only 50% to 70% of VT, with focal mechanisms and bundle branch reentry comprising the remainder (60,61). Most patients have multiple distinct QRS morphologies, often with different mechanisms. Given the foregoing considerations, it is not surprising that programmed stimulation is less often successful in inducing clinical VT. In addition, early series of endocardial ablation in these patients had low success rates.
A simple and safe technique for percutaneous subxiphoid pericardial instrumentation has been introduced (62). Preliminary studies confirmed the extensive nature of epicardial scar in NCD and demonstrated successful ablation of epicardial foci or slow conduction zones not amenable to ablation from the endocardium (63,64). These techniques, combined with greater use of substrate-based approaches to ablation, have improved the overall outcome of catheter ablation in this population. The efficacy and survival benefit of ICDs for patients with sustained VT appear similar to those in patients with ischemic heart disease, and this approach recommended as primary therapy in most patients.
Right Ventricular Cardiomyopathy/Dysplasia
Right ventricular cardiomyopathy/dysplasia (ARVD) is a heart muscle disease characterized by progressive myocyte loss and
fibrofatty replacement, with a predilection for the right ventricle. It is familial in 30% to 50% of cases, with a majority demonstrating an autosomal dominant pattern with incomplete penetrance. Causative mutations have been identified in plakoglobin, desmoplakin, and plakophilin, all of which encode major components of the desmosome (65). These protein complexes anchor intermediate filaments to the cytoplasmic membrane and form a three-dimensional scaffolding that provides mechanical strength. Impaired functioning of cell adhesion junctions during exposure to shear stress may lead to myocyte detachment and death, accompanied by inflammation and fibrofatty repair. In addition, disruption of cell adhesion junctions leads to gap junction remodeling and reduced cell–cell electrical coupling (66). The mechanism for VT is nearly always reentry, with surviving muscle bundles with impaired electrical coupling serving as sites of slow conduction analogous to postinfarction VT.
fibrofatty replacement, with a predilection for the right ventricle. It is familial in 30% to 50% of cases, with a majority demonstrating an autosomal dominant pattern with incomplete penetrance. Causative mutations have been identified in plakoglobin, desmoplakin, and plakophilin, all of which encode major components of the desmosome (65). These protein complexes anchor intermediate filaments to the cytoplasmic membrane and form a three-dimensional scaffolding that provides mechanical strength. Impaired functioning of cell adhesion junctions during exposure to shear stress may lead to myocyte detachment and death, accompanied by inflammation and fibrofatty repair. In addition, disruption of cell adhesion junctions leads to gap junction remodeling and reduced cell–cell electrical coupling (66). The mechanism for VT is nearly always reentry, with surviving muscle bundles with impaired electrical coupling serving as sites of slow conduction analogous to postinfarction VT.
The process may initially be focal, preferentially affecting one or more of the anterior infundibulum, apex, and inflow tract (so-called “triangle of dysplasia”). Imaging techniques demonstrate structural and functional abnormalities including localized akinetic or dyskinetic bulges or aneurysms or more generalized akinesia and dilation (67). Global left ventricular function is generally normal, although histologic evidence of left ventricular involvement is present in up to 50% of cases. ECG abnormalities (T-wave inversion, QRS duration ≥110 milliseconds, S-wave upstroke ≥55 milliseconds in leads V1 to V3) are present in 80% to 90% of patients with confirmed disease (68), and they may precede functional abnormalities. Diagnostic criteria have been formulated that incorporate major clinical features and laboratory abnormalities, although the criteria may be less sensitive for early disease (69) (Table 66.3). However, excessive reliance on the nonspecific findings of imaging tests may lead to overdiagnosis (70).
Monomorphic VT is the most common sustained arrhythmia in ARVD, with peak presentation in the third and fourth decade of life (71,72,73,74). A minority of patients may present with polymorphic VT or VF. Recurrence of VT is common and often occurs in bursts with relatively long periods of quiescence. VT nearly always has a left bundle pattern, with the frontal plane axis equally divided between inferior and left superior. Multiple morphologically distinct tachycardias are present in up to 50% of patients. Consistent with a reentrant mechanism, VT can usually be induced during programmed stimulation in patients presenting with spontaneous tachycardia. Low voltage, fragmented, or delayed sinus rhythm endocardial and epicardial electrograms are often recorded from involved myocardium (75). This arrhythmogenic substrate may be detected by an abnormal signal-averaged ECG in up to 70% of patients.
Long-term prognosis is excellent in patients with ARVD and VT treated with ICDs (74,76). Device therapy also offers the potential advantage of pace termination, thus avoiding the need for long-term antiarrhythmic drugs. Drug therapy is empiric, with β-blockers, sotalol, and amiodarone all of potential utility in patients with frequent episodes. The acute success rate of catheter ablation appears is high (80%); however, there is a significant incidence of late recurrence, often the result of new foci, most likely reflecting the progressive nature of this disease (77,78). For this reason, ablation as primary therapy without ICD backup remains controversial.
Hypertrophic Cardiomyopathy
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree