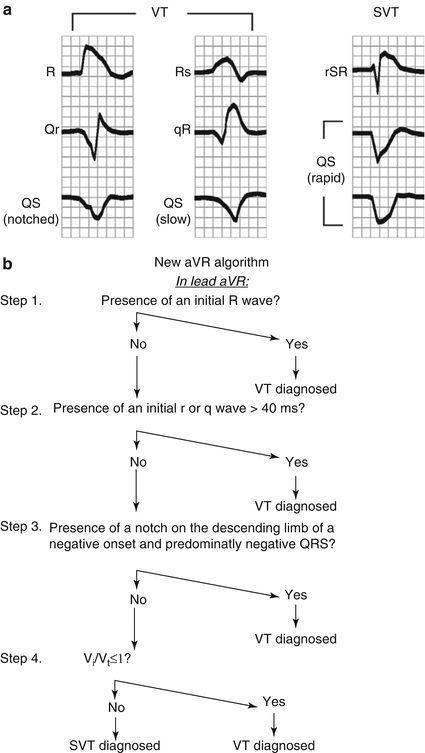
Figure 7.2
Algorithm to determine VT vs SVT with aberrancy by criteria developed in aVR. (a) By aVR QRS morphologies. (b) Algorithm decision ladder (Used with permission)
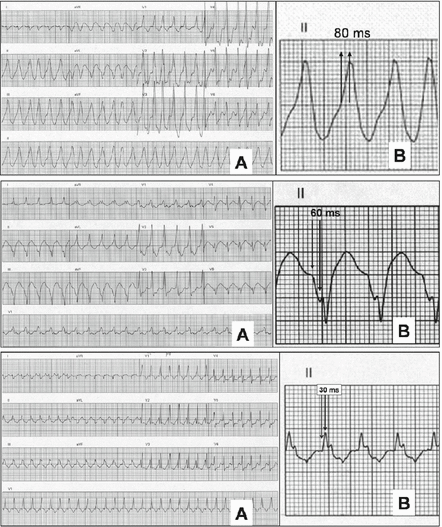
Figure 7.3
Algorithm to determine VT vs. SVT by measuring the duration of onset of QRS to the peak of R wave lead II. Signals measuring ≥50 ms (top two panels) suggest VT whereas <50 ms suggest SVT with aberrancy [4] (Used with permission)
Once diagnosis is made that the arrhythmia is VT, the next step is to determine the activation vector of the ventricle. As a simplistic starting point, the bundle branch block appearance of the QRS complex indicates the culprit chamber where the VT is originating from. A left bundle branch block appearance indicates an RV or septal LV VT while a right bundle branch appearance indicates an LV VT origin. Taking this approach a step further, using the right sided leads (V1, AVR), inferior leads (II/III/AVF), lateral leads (1/AVL) and apical leads (V5/V6) one can identify where the VT is coming from (negative QS vector) and going towards (positive RS vector). Generally, biphasic QRS vectors mean that the origin is somewhere in the middle of that individual vector, i.e. a biphasic QRS vector in 1 and V1 likely indicate that the origin/exit site of the VT is in the septum and not on the right (RV, Q wave in V1) or left (posterior LV, Q wave in 1). This approach is primarily useful in reentrant VT’s and idiopathic PVC’s.
A close examination of the resting 12 lead ECG can also provide significant clues as to the underlying process and likely culprit areas of myocardial scarring. The presence of Q waves in the distribution of a coronary artery should indicate the presence of scarring that will often play a critical role in sustaining VT. Fragmentation (extra notching) of the surface QRS complex in a similar distribution to a major coronary vessel and Q waves often can provide a more sensitive indicator of myocardial scarring and may indicate the presence of epicardial scarring in that region.
For idiopathic VT’s such as RVOT and LVOT VT, the appearance should be consistent with an outflow origin (inferior axis, i.e., positive in the inferior leads II/III/AVF) with an earlier precordial transition (V3 or less) indicating an LV origin and a later transition (V3 or later) indicating an RV origin. Transition at V3 could represent either LV or RV origin. Idiopathic VT utilizing the conduction system (bundle branch and fasicular reentry VT’s) are quite uncommon but do have characteristic features that should set them aside from VT associated with structural heart disease. The VT is generally slower with a typical bundle branch or fasicular block appearance during tachycardia that is usually very similar to the appearance of the baseline QRS complex at rest Figure 7.12. Bundle branch and fasicular reentry are usually associated with baseline conduction disease, including bundle branch block of either right or left and prolonged AV or PR interval, with the absence of such on a resting 12 lead ECG making them very unlikely to be the mechanism for the VT.
Scar Mediated Ventricular Tachycardia
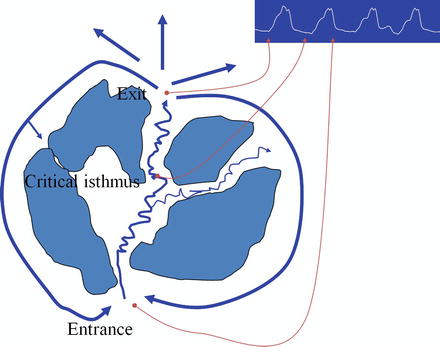
Within westernized cultures the most common underlying cause of recurrent VT is remote coronary disease. Secondary to a focus on shorter door to balloon times for the acute myocardial infarction patient, more patients are surviving their myocardial infarctions only to eventually experience downstream congestive heart failure and ventricular tachycardia. The ischemic insult of a myocardial infarction results in the formation of myocardial scarring with variable transmural extent and resulting in altered electrical conduction within the infarcted myocardial region. Electrical conduction through scar is delayed and the resultant zone of slow conduction represents one of the critical elements of the reentrant circuit. This along with differential refractory periods in the surrounding tissue comprise the necessary substrate to sustain VT [5]. Ventricular ectopy is often the inciting event that initiates the ventricular tachyarrhythmia, either VT or VF. Premature ventricular contractions are sent through the slow conduction zone present within the ventricular scar with sufficient delay to result in their exit on the opposite side of the scarred region, finding the myocardium ready for depolarization. The electrical wave propagates around the more dense areas of scarring or anatomical barrier, such as heart valve annulus, and back into the entrance of the zone of slow conduction through the scar, creating a figure of eight pattern with the critical isthmus representing the central portion of the figure of eight (Fig. 7.4).
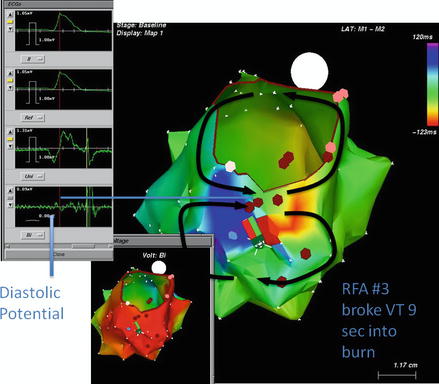

Figure 7.4
Figure of eight reentrant ventricular tachycardia circuit encircling around a dense inferoposterior left ventricular scar and the mitral valve annulus. The central portion of the figure of eight comprises the critical isthmus with a zone of slow conduction and the area where diastolic potentials may be recorded. This site, following confirmation with pacing maneuvers, often is the target of successful ablation
The typical ischemic ventricular tachycardia patient will present to medical care with either recurrent defibrillator therapies (shocks) or recurrent presyncope. Presyncope VT patients are often being treated with antitachycardia pacing and thus being prevented from progressing to either shocks or actual syncope. Of concern is the patient that presents with incessant VT or frequent – defined as >3 episodes of VT within 24 hours. This is termed VT storm and often requires multiple shocks and acute inpatient admission for management, accompanied by a significant rise in both inpatient and short term mortality [6]. The management of all of these patients is similar with a few exceptions made for the VT storm patient. In general, recurrent antitachycardia pacing or lone outpatient ICD shocks should result in the initiation or acceleration of antiarrhythmic drug therapy, starting with sotalol or amiodarone, followed by the addition of mexiletine if necessary. It is also important to make sure that these patients are adequately beta blocked since the majority of ischemic VT’s are going to possess an adrenergic component and be beneficially treated with beta blockade. Once patients experience recurrent ICD therapies for VT while on an appropriate antiarrhythmic drug regimen, ventricular tachycardia ablation should be considered.
The management of VT storm patients is more difficult and varied than the ambulatory ischemic cardiomyopathy patient with recurrent ATP or lone ICD shocks for ventricular tachycardia. VT storm patients who present with recurrent ICD shocks often are accompanied by hemodynamic instability. The first step in their management is to establish control of their ventricular rhythm. Intravenous antiarrhythmic agents are utilized acutely to achieve this with both amiodarone and lidocaine used commonly. Although limited by toxicity, lidocaine can be a useful agent especially if there are concerns for ongoing ischemia. However, a combination of amiodarone (or sotalol) and lidocaine is often required to achieve control of the malignant ventricular arrhythmias in this situation. In addition to antiarrhythmic drug therapy and aggressive beta blockade, sedation and sometimes intubation are utilized to minimize adrenergic contribution to the VT. As an illustrative point to the contribution of sympathetic tone to the generation of recurrent VT in patients with an abnormal LVEF, bilateral sympathectomy has been recently shown to be effective for the suppression of acute ICD therapies for VT storm patients and has displayed durability over medium term follow up [7].
Following the initiation of intravenous antiarrhythmic drug therapy, ongoing myocardial ischemia should be considered and evaluated, likely with coronary angiography. If myocardial ischemia is present and is the main substrate of the VT, successful treatment with revascularization often subsides the VT. However, it is usually required to accelerate the patient’s oral antiarrhythmic drug therapy prior to discharge and to establish short term follow up to make sure that the treated lesion was indeed the ischemic driver of the VT.
For VT storm patients or ambulatory patients with recurrent VT despite antiarrhythmic drug therapy, electrophysiologic testing and ablation of their VT should be considered. VT ablation has evolved since its inception to a well defined repeatable process designed to identify electrical scar, the culprit ventricular tachycardia and its dependent isthmus.
Electrophysiologic Evaluation of VT with Structural Heart Disease
For patients being evaluated for EP testing and ablation of a ventricular arrhythmia it is important to provide the appropriate setting. The use of general anesthesia for these procedures has become standard secondary to the length of the procedure and the risk for multiple defibrillations. Three dimensional mapping systems have also become standard and represent one of the most powerful tools to allow an in depth study of the ventricular chamber in question and increase the efficacy of VT ablation.
Prior to the procedure, the patient should be thoroughly assessed regarding the appropriate approach to the chamber in question. Generally the right ventricle does not pose much of a challenge outside of patients with complex congenital heart disease or tricuspid valve surgery. However, the left ventricle may prove inaccessible from a retroaortic or transseptal approach due to the presence of mechanical aortic or mitral valves. The location of culprit scar is also an important consideration as posterior, inferior and lateral scars are more easily accessible from a transseptal approach while LVOT, and anterior and septal scars are via a retroaortic approach. Peripheral arterial disease can make a retro aortic approach difficult if not impossible, which should prompt the performance of a peripheral arterial exam (bruits and pulses) prior to procedure onset. In addition, the ability to access the epicardial space (the absence of a prior sternotomy) should also be considered at procedure onset to determine if it is an option if required.
During procedure onset and throughout the duration, control of the patient’s bradyarrhythmia should be available through the implanted device (in the likely event that it is present). It is best to leave patients that are not ventricular pacing dependent paced in the atrium only to allow intrinsic conduction for more accurate endocardial scar mapping. For ventricular pacing dependent patients it is often necessary to leave biventricular pacing in place to maintain appropriate hemodynamic status for the purpose of anesthesia. It may also be necessary to review the induced ventricular tachycardia on the far field electrogram through the device, which is only possible if the programmer is on and communicating with the device. The implanted device can also provide a means of intracardiac defibrillation if such a rescue is needed.
Once access to the LV endocardium or epicardium has been obtained, electrophysiologic study and ablation of ventricular tachycardia proceeds in five steps: (1) anatomic definition; (2) endocardial scar mapping; (3) induction and study of ventricular tachycardia, including entrainment mapping; (4) ablation of ventricular tachycardia, late potentials within scar; (5) attempt reinduction of ventricular tachycardia. With abolition of the clinical ventricular tachycardia and inability to induce anything other than ventricular fibrillation (rate >200 bpm) the study is complete.
To define the left ventricular anatomy a three dimensional map is generated with the aid of either fluoroscopy or ultrasound, and often both. Other than the geometry of the left ventricle itself, this often includes structures such as the mitral annulus, left ventricular outflow tract, left sided His bundle, Purkinje potentials and papillary muscles. It is important to make sure that the anatomy collected is complete. Correspondingly, the right ventricular structures including the AV node and the His conduction system, tricuspid valve, pulmonic valve, right ventricular outflow tract should be noted. Generally the risk of right ventricular perforation is higher and thus more care during mapping is needed. As the definition of endocardial scarring is dependent upon catheter contact with the wall, it is essential to know if contact is indeed present; otherwise areas will be labeled as scar inappropriately. This has been aided with real time two dimensional intracardiac echocardiography along with the evolution of contact force catheters, both of which can be used to provide definitive evidence of endocardial contact. Multi electrode mapping catheters are commonly utilized to collect a significantly larger amount of data in a shorter period of time both regarding anatomy and endocardial voltage.
Generally at the same time that anatomic information is collected, voltage is collected to provide visual information of the distribution of endocardial scarring relative to the anatomy collected. The definition of endocardial scar varies somewhat but is generally accepted to be any myocardial tissue with less than 0.5 mv. In similar fashion to the necessity of complete anatomic collection, it is just as important to generate a complete detailed voltage map. With identification of the scar, additional focus should be placed upon the scar itself for either fragmented potentials or late potentials most recently coined to be late areas of ventricular activation or LAVA. LAVA are characterized by high frequency or fragmented signals that can be either late relative to the QRS complex and local signal or buried completely within the local signal (Fig. 7.5).
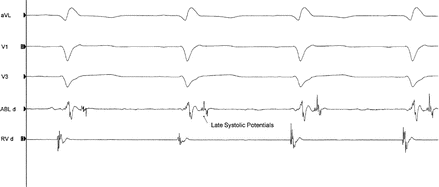

Figure 7.5
Late systolic potentials noted during substrate ventricular mapping recorded in sinus rhythm
In addition, areas that LV capture may be generated from within the endocardial scar are suspicious for possible VT isthmus and should be tagged for eventual ablation.
With a complete endocardial scar map, the next step within our lab is to induce ventricular tachycardia via programmed stimulation via a quadripolar pacing catheter in the RV. A predominantly substrate based approach does not involve induction of VT and ablation is carried out with the intention to abolish all LAVA within and around the endocardial scar. At this point it is crucial to reference the tachycardia 12-lead ECG for the rate and appearance of the VT, if available. Within the defibrillator population one may not ever have a surface electrocardiogram as the majority of their events are treated prior to presentation to medical care via their device. As a result only the cycle length and far field electrogram through the device are available to correlate the VT induced during the procedure with the clinical VT.
Once VT is induced matching the clinical VT the approach to study of the VT can vary from patient to patient. If the VT is hemodynamically tolerable (most often seen with slower VT’s [<150 bpm] in patients with only moderate LV dysfunction) then activation and entrainment mapping may be pursued. If not hemodynamically tolerable, then the VT is pace terminated or terminated via defibrillation and pace mapping to define the VT isthmus based upon the VT morphology followed by a LAVA ablation strategy is appropriate. Both situations will be discussed in the following sections.
The Study of Hemodynamically Tolerable VT
Hemodynamically tolerable VT has become increasingly uncommon within the electrophysiology lab. Most of the VT’s that are being mapped currently are fast VT’s in patients with more advanced LV dysfunction. It may often be necessary to utilize vasopressors or in some cases device based hemodynamic support (Intra-aortic balloon pump, percutaneous left ventricular assist device such as Impella or ECMO) to provide adequate perfusion pressure in an effort to allow mapping. Goal blood pressures should be a mean arterial pressure above 50 mmHg.
When VT is tolerable it is beneficial to perform activation and entrainment mapping to confidently abolish the clinical VT. Points are taken and recorded via the mapping system to identify early and late EGM’s with the area in the scar connecting early to late representing the slow component of the VT circuit and the critical isthmus. Within the early meets late area entrainment via the mapping catheter is performed by pacing 10–20 ms faster than the VT. Capture is maintained for sufficient time to overtake the VT circuit (5–10 beats) followed by cessation. If the morphology of the tachycardia changes during pacing, termed “manifest entrainment”, and is associated with a post pacing interval (PPI) longer than the tachycardia cycle length then the pacing site is from an area outside of the tachycardia circuit. Ablation at these sites will not result in termination of VT. Suitable targets for successful ablation should results in “concealed entrainment”. Pacing from these sites results in acceleration and exact morphology match as the tachycardia. Sites associated with the highest short and long term success in eliminating the VT circuit with ablation are in the critical isthmus. In addition to concealed entrainment, pacing from within the critical isthmus, the difference between the PPI and the tachycardia cycle length (TCL) will be small (likely less than 20 ms), and recording of presystolic potentials with an activation time to surface QRS similar to stimulus to surface QRS (also less than 20 ms) indicate that the mapping catheter is located within the VT critical isthmus (Fig. 7.6).
 < div class='tao-gold-member'>
< div class='tao-gold-member'>





Only gold members can continue reading. Log In or Register to continue
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


