Figure 4.1
Surface ECG morphologies from a patient with WPW in different situations. (a) Sinus rhythm with subtle preexcitation, (b) Preexcitation maximized by slow atrial pacing plus adenosine, (c) Preexcitation maximized by rapid atrial pacing, (d) Irregular rhythm with variable degrees of preexcitation due to atrial fibrillation, (e) Regular rhythm with maximal preexcitation due to antidromic AVRT
The diagnosis of WPW is generally straightforward from the baseline ECG. Occasionally fusion between sinus and ventricular beats falsely gives the appearance of preexcitation (for example, late-coupled ventricular bigeminy can look like preexcitation alternans). Preexcitation can sometime be subtle (see Figs. 4.1a and 4.2), due to variable degrees of fusion between antegrade nodal and antegrade pathway conduction. Subtle preexcitation is more likely when the atrial depolarization reaches the pathway late (i.e. left-sided pathways), when nodal conduction is relatively rapid (i.e. young patient, elevated sympathetic tone), or when pathway conduction velocity is slow (particularly certain types of atypical pathways, discussed elsewhere). Antegrade pathway conduction can be absent or intermittent above certain atrial rates, depending on the effective refractory period of the pathway. Antegrade pathway conduction can also be absent for reasons unrelated to pathway refractoriness (so-called “latent” pathways), including the phenomenon of concealed penetration of the His-Purkinje wavefront retrograde into the pathway (see Fig. 4.3a).
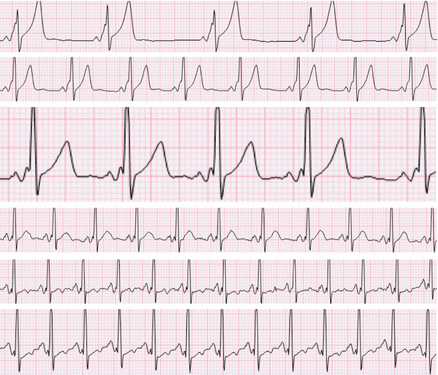
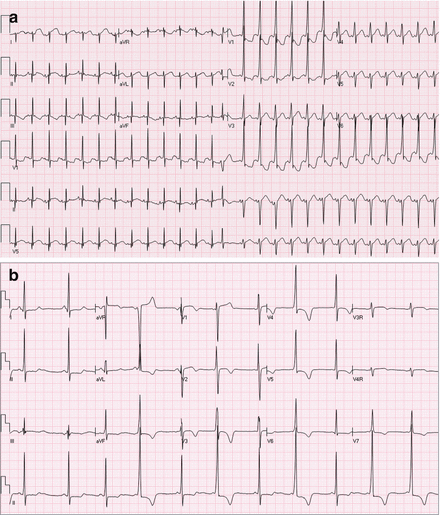

Figure 4.2
Series of strips from a single patient at various sinus rates, demonstrating variable degrees of fusion (related to autonomic effects on AV node conduction time, with antegrade pathway conduction present throughout); it is incorrect to use this type of “loss of preexcitation at faster heart rates” as a sign of a low-risk pathway

Figure 4.3
(a) Apparent “intermittent” preexcitation in a child with WPW, with narrow QRS due to repetitive concealed penetration of the His-Purkinje wavefront retrograde into the pathway, until a PAC disturbs the pattern and allows antegrade pathway conduction at the same sinus rate; this patient had “high-risk features” demonstrated at EP study. (b) Truly intermittent preexcitation (on a beat-by-beat basis) in a different patient with WPW and hypertrophic cardiomyopathy; note half-scale voltage for precordial leads
Additional rhythm recording (Holter, event recorder) may be indicated depending on symptoms. Echocardiography is indicated at baseline (to evaluate for comorbid structural heart disease, including Ebstein anomaly of the tricuspid valve, other forms of congenital heart disease, and hypertrophic cardiomyopathy) and perhaps periodically thereafter (to evaluate for ventricular dysfunction as mentioned above). Family screening is not generally recommended, as the recurrence risk in relatives is only modestly elevated (although certainly families with multiple affected members do occur).
Finally, the possibility of risk for sudden death must be considered. The mechanism of sudden death is believed to be that of rapidly conducted atrial fibrillation resulting in rapid irregular ventricular stimulation and eventually ventricular fibrillation. Patients with WPW are at increased risk for atrial fibrillation, even in the absence of structural heart disease. Therefore the risk of sudden death depends primarily on the antegrade conduction characteristics of the pathway. Patients with syncope, documented preexcited atrial fibrillation, multiple preexcited morphologies (since multiple pathways may alternate to allow rapid ventricular stimulation), and SVT (especially antidromic SVT) are at increased risk. Generally, older patients are believed to be at decreased risk, presumably because of survivor bias (i.e. those with rapid antegrade conduction would have presented with syncope or sudden death already).
A consensus statement from PACES (the Pediatric and Congenital EP Society) and HRS (the Heart Rhythm Society) provides guidance for the management of asymptomatic WPW in children and young adults [1]. Although studies differ about the exact magnitude of the risk for sudden death (e.g. between 0.1 and 5 per 1,000 patient-years), the guideline authors provide class IIa recommendations for risk stratification (acknowledging that no patient is at zero risk). The first step is non-invasive risk stratification, including ECG and if necessary exercise stress test, with the goal of identifying “abrupt and complete” loss of preexcitation at faster heart rates (felt to predict long accessory pathway refractory period). It is important to include only abrupt loss (i.e., in a single beat, see Figs. 4.3b and 4.4), and not be misled by gradual fusion or day-to-day variation due to concealed retrograde penetration (see Figs. 4.2 and 4.3a). Only 10–20 % of patients will have convincingly reassuring non-invasive evaluations; invasive testing is recommended for the remainder. Invasive testing consists of diagnostic EP pacing study via either trans-esophageal or trans-venous route, with the goal being to induce sustained atrial fibrillation and measure the SPERRI (Shortest Pre-Excited R-R Interval, see Fig. 4.5). Assessment of SPERRI during atrial fibrillation is a stronger predictor than other pathway characteristics (such as SPERRI during rapid atrial pacing, or accessory pathway ERP, see Fig. 4.6) and should always be sought. Atrial fibrillation can be induced, with concerted effort, in well over 90 % of patients with WPW; prolonged rapid pacing just above atrial refractoriness usually suffices, but ramp pacing protocols and administration of adenosine can be helpful in difficult cases. SPERRI < 250 ms, and especially <220 ms, is a predictor of higher risk for sudden death, and therefore an indication for prophylactic ablation (class IIa). The full algorithm (see Fig. 4.7) and accompanying text [1] capture additional nuance about balancing risks and benefits of ablation vs conservative management in various clinical situations.
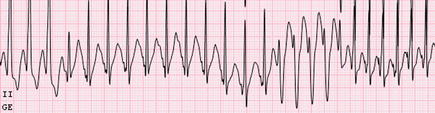
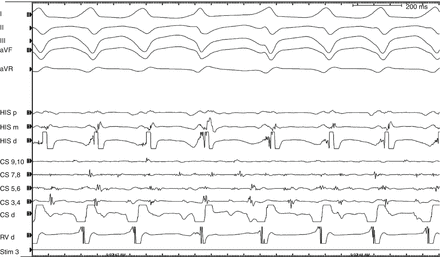
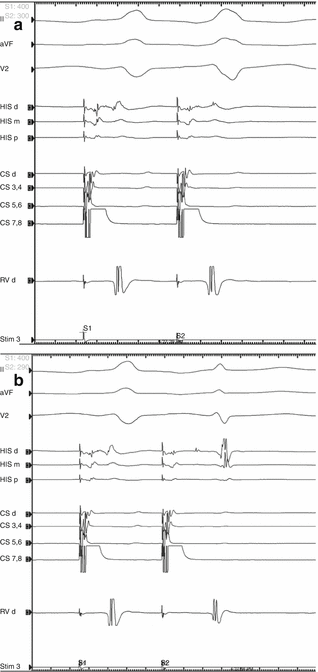
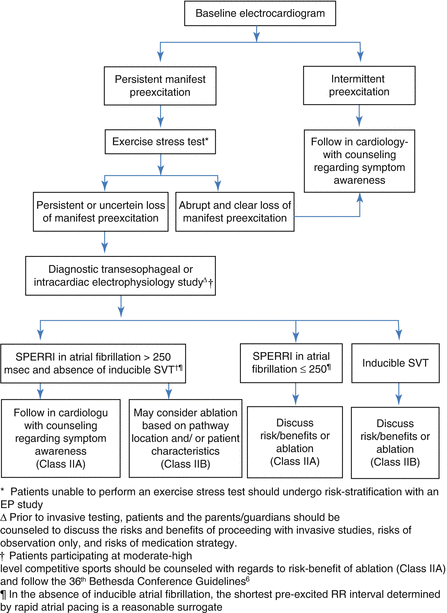

Figure 4.4
Strip from a patient with WPW undergoing exercise stress test. Preexcitation was present for the majority of the test, up through the first 3 beats on this strip. Then there is abrupt loss of preexcitation (in a single beat, associated with clear increase in PR) in the setting of sinus tachycardia to just over 200 bpm. After 11 beats of sinus tachycardia with antegrade nodal conduction, SVT begins, at a rate of nearly 300 bpm, with rate-related aberrancy for the first 4 beats

Figure 4.5
Atrial fibrillation with rapid antegrade pathway conduction; the shortest preexcited RR interval was 212 ms, suggestive of elevated risk for sudden death

Figure 4.6
Surface and intracardiac electrograms from atrial extrastimuli testing showing the accessory pathway effective refractory period with drive train S1 cycle length 400 ms and (a) S2 = 300 ms resulting in simultaneous antegrade pathway and nodal conduction with H-V interval < 0, compared to (b) S2 = 290 ms resulting in exclusively nodal conduction, with a much longer PR interval, normal H-V interval, and narrow QRS

Figure 4.7
Flowchart from PACES/HRS guidelines1 for risk stratification of asymptomatic children and young adults with WPW (Reprinted with permission)
Pharmacologic Management
For patients with WPW, medication is generally reserved for management of SVT (orthodromic AVRT) (Fig. 4.8) in patients felt not to be at risk for sudden death or who decline ablation. Because of some evidence that susceptibility to AVRT is itself a predictor of elevated risk (perhaps because rapid atrial stimulation during SVT can promote atrial fibrillation), ablation is used fairly liberally for patients with WPW and SVT. It is unknown if the risk stratification criteria for asymptomatic patients (see Fig. 4.7) can be extrapolated to patients symptomatic with palpitations or SVT.
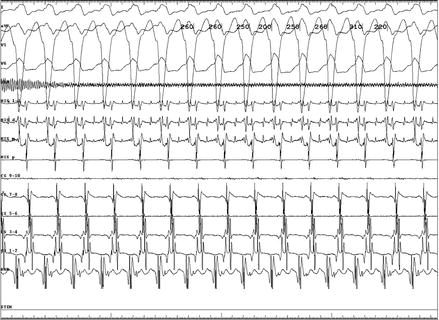

Figure 4.8
Orthodromic AVRT with LBBB aberrancy and earliest atrial activation at the His position, in a patient with an anteroseptal pathway
For management of AVRT, unlike in patients with concealed accessory pathways (see elsewhere), digoxin and calcium-channel blockers are considered contraindicated in patients with WPW because of an association with sudden death (AV node blockade is felt to promote antegrade conduction down the pathway). Although the same concern theoretically exists for beta-blockers, these medications have been used extensively in patients with WPW without convincing evidence for untoward effects. However, it is important to realize that beta-blockade does not reliably protect against rapidly conducted atrial fibrillation and sudden death in predisposed patients. Therefore beta-blockers are primarily appropriate for management of SVT in patients felt to be at low risk for malignant arrhythmia.
< div class='tao-gold-member'>
Only gold members can continue reading. Log In or Register to continue
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


