Ventricular septal defect (VSD), the most common form of congenital heart disease, is frequently seen by both the adult and pediatric echocardiographer. Fifty percent of all children with congenital heart disease have an associated VSD, with 20% of cases being isolated VSDs. VSDs are an integral part in many forms of congenital heart disease, including tetralogy of Fallot, truncus arteriosus, and double-outlet right ventricle. The incidence of an isolated VSD in live births is 1.5 to 53 per 1000, with wide variation in reported incidence rates.
As in most congenital heart lesions, echocardiography has become the mainstay for clinical diagnosis, for direction of clinical care, and for determination of the approach to therapeutic intervention. Thus, while the presence of a VSD is often easily appreciated, ideal echocardiographic study and interpretation require that the echocardiographer have a detailed understanding of septal anatomy, relationships of VSDs to other intracardiac structures, and the impact of these VSDs on intracardiac hemodynamics.
ANATOMY OF THE VENTRICULAR SEPTUM
The normal ventricular septum is a curved structure extending from the posterior interventricular groove at its inferior and rightward aspect to the pulmonary outflow tract and anterior interventricular groove superiorly and leftward. The ventricular septum can be divided into four regions: the membranous, inlet, outlet, and trabecular septa (Fig. 11.1). The borders of these regions are determined by the tricuspid, pulmonary, and aortic valves, with subdivisions of the regions determined by the muscular bands in the right ventricle: the septal, parietal, and moderator bands. Of the muscular bands, only the moderator band is easily appreciated by standard two-dimensional echocardiography. This muscular bundle originates from the right ventricular (RV) side of the mid-septum at its apical third and crosses the RV chamber to the parietal wall. Also called the trabeculum septomarginalis, the septal band is difficult to appreciate echocardiographically. It is a muscular ridge extending along the RV side of the mid-septum from the insertion of the moderator band, toward the aortic outflow where it bifurcates into an anterior and posterior limb. Within this bifurcation are the membranous septum and the subpulmonary outlet septum.
The membranous septum is a small fibrous portion of the ventricular and atrioventricular (AV) septum located at the base of the heart, adjacent to the anteroseptal tricuspid commissure, the right posterior aortic valve commissure, and the anterior mitral valve leaflet. Because of the relative apical placement of the tricuspid valve compared with the mitral valve, a portion of the membranous septum, the membranous AV septum, separates the left ventricle (LV) from the right atrium.
The three other regions of the ventricular septum are all muscular; they radiate out from the membranous septum. The inlet septum is located between the AV valves inferior to the membranous septum with its apical border being the chordal attachments of the AV valves. The outlet septum makes up the most anterior and superior part of the ventricular septum and is located above an imaginary line between the membranous septum, the papillary muscle of the conus, and the anterior infundibular wall. The remainder of the ventricular septum is the trabecular septum, which is the largest region. The trabecular septum is broken into the subregions shown in Fig. 11.2: posterior (sometimes called inlet muscular), anterior, mid-muscular, and apical. The posterior trabecular (or muscular) septum is posterior to the septal attachment of the tricuspid valve. The anterior trabecular septum is anterior to the septal band (or trabeculum septomarginalis). As the septal band is difficult to appreciate by echocardiography, the anterior trabecular septum is identified as anterior to the mid-septum and at, or superior to, the level of the moderator band. The mid-muscular septum is posterior to the septal band and superior to the moderator band. The apical septum is inferior to the moderator band and can be divided into an anterior or “infundibular” apex and posterior or “inflow” apex.
ANATOMY OF VENTRICULAR SEPTAL DEFECTS
VSDs can be divided into two fundamental types. In the first type, there may be adequate septal tissue, but there is malalignment of portions of the ventricular septum causing a “gap” or VSD. The malaligned septal components can be parallel but offset, can cross each other in oblique planes, or even can be perpendicular to each other. The second fundamental type of VSD is due to a deficiency in septal tissue. This deficiency either can be congenital or can be acquired following myocardial infarction or trauma.
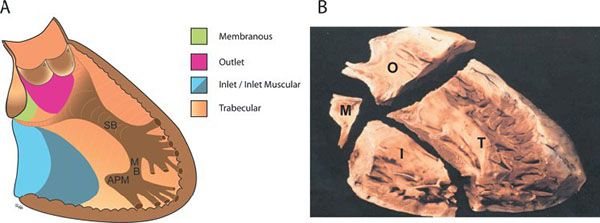
Figure 11.1. Diagrammatic (A) and pathologic (B) representations of the normal interventricular septum as viewed from the right ventricular aspect. APM, anterior papillary muscle of the tricuspid valve; I, inlet; M, membranous; MB, moderator band; O, outlet; SB, septal band; T, trabecular. (B, Reprinted with permission from Becker A, Anderson R. Anomalies of the ventricles. In: Cardiac Pathology and Integrated Text and Colour Atlas. New York: Raven Press, 1983:12.2.)
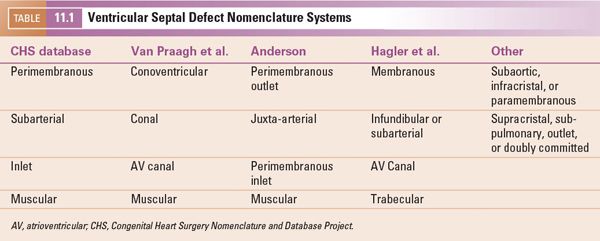
Several classification schemes to describe the location of VSDs are in common use. Thus, the nomenclature can be quite confusing. Efforts to synthesize the various naming systems have been made by the Congenital Heart Surgery Nomenclature and Database Project and subsequently by the International Working Group for Mapping and Coding of Nomenclatures for Pediatric and Congenital Heart Disease. The classification systems most commonly used are shown in Table 11.1. We use the Congenital Heart Surgery Nomenclature system in this chapter and have chosen to use the following names: perimembranous (including VSDs due to malalignment of the conal septum), subarterial, inlet, and muscular. Defects can occur entirely within a portion of the septum or can extend across portions of the septum (e.g., a perimembranous-to-inlet defect). Regardless of which naming system is used, it is most important to be consistent so that accurate and clear communication can occur.
ECHOCARDIOGRAPHIC ASSESSMENT OF VENTRICULAR SEPTAL DEFECTS
A systematic echocardiographic assessment of VSDs involves a detailed anatomic and hemodynamic description. This includes description of the exact location of the defect in the ventricular septum with particular attention paid to (a) the relationship of the VSD to valves and valve attachments, (b) identification of complicating factors specific to the VSD location, (c) description of the anatomic size of the defect, (d) estimation of right ventricular systolic pressures, and (e) estimation of overall shunt size.
The general imaging strategy used to identify and describe VSDs consists of sweeping the entire septum in both two-dimensional and color Doppler imaging modalities from apex to base and from left to right. Imaging should be performed from the best acoustic window that shows the septum perpendicular to the ultrasound beam and the flow across the defect parallel to the beam. Because of the curved nature of the ventricular septum, optimal imaging of a VSD can be from a subcostal, parasternal, apical, or right parasternal window. It often requires imaging from multiple planes to fully interrogate the ventricular septum.
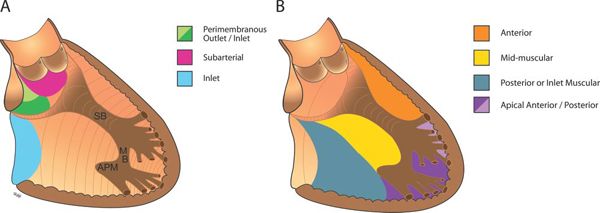
Figure 11.2. Location of ventricular septal defects (VSDs) within the ventricular septum as seen from the right ventricular aspect. A: Locations of perimembranous, subarterial, and inlet VSDs. B: Subtypes of muscular VSDs: anterior, mid-muscular, posterior, and apical (including VSDs of the posterior right ventricular [RV] inflow apex and the anterior infundibular apex). APM, anterior papillary muscle of the tricuspid valve; MB, moderator band; SB, septal band.
Description of Ventricular Septal Defect Location
The exact location of the VSD can be identified echocardiographically and described using the categories described earlier (perimembranous, subarterial, inlet, or muscular). Figure 11.3 is a diagrammatic representation of the various types of defects and their location in standard echocardiographic views. Identification of the location of the major portion of the defect should be accompanied by descriptions of extension of the defect into adjacent portions of the ventricular septum and the direction of malalignment of the septum. Defects in larger regions, such as the trabecular septum, often need to be more precisely located by describing the relationship to adjacent cardiac structures. The presence or absence of specific complicating factors associated with each VSD location should also be evaluated and reported.
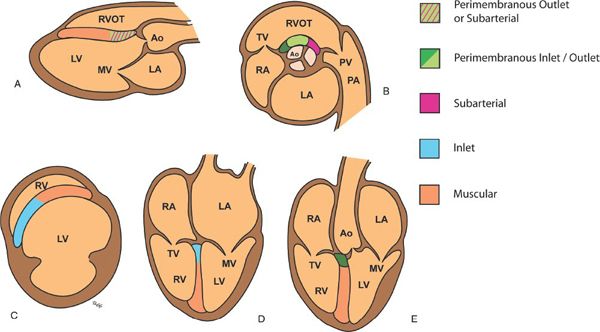
Figure 11.3. Diagrammatic representation of ventricular septal defect (VSDs) locations as seen in standard echocardiographic views. A: Parasternal long-axis view showing muscular, perimembranous outlet, and subarterial VSDs. B: Parasternal short-axis view at the base showing perimembranous and subarterial VSDs. C: Parasternal short-axis view at the level of the left ventricular (LV) papillary muscles showing muscular VSDs. D: Apical four-chamber view showing inlet and muscular VSDs. E: Apical five-chamber view showing muscular and perimembranous VSDs. Ao, aorta; LA, left atrium; MV, mitral valve; PA, pulmonary artery; PV, pulmonary valve; RA, right atrium; RV, right ventricle; TV, tricuspid valve.
Perimembranous VSDs
Perimembranous defects are the most common type of VSD, comprising about 80% of VSDs. Perimembranous VSDs involve the membranous ventricular septum adjacent to the aortic and tricuspid valves. Perimembranous defects can be imaged from a parasternal, apical five-chamber, or subcostal window where they are seen immediately adjacent to both the tricuspid and aortic valves as demonstrated in Figure 11.4 When the VSD extends primarily toward the aortic valve, it is called a perimembranous outlet defect; when the defect is primarily adjacent to the tricuspid valve, it is called a perimembranous inlet defect.
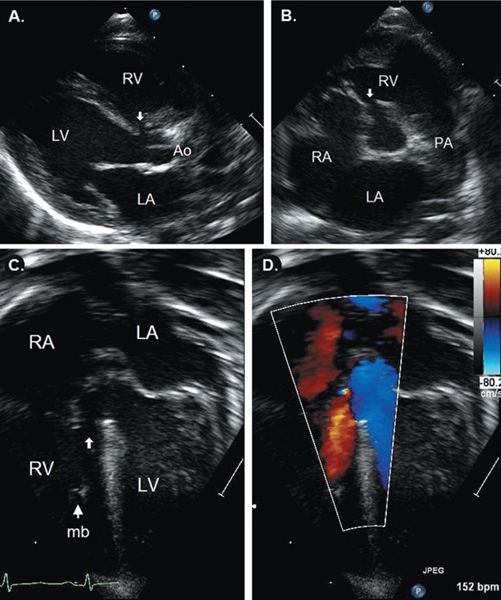
FIGURE 11.4. Perimembranous ventricular septal defect (VSD) (arrows). A: Parasternal long-axis showing the VSD adjacent to the aortic valve. B: Parasternal short-axis image showing defect between aortic and tricuspid valves. Apical five-chamber view with two-dimensional (C) and color (D) images showing relationship of VSD to left ventricular (LV) outflow tract. Ao, aorta; LA, left atrium; LV, left ventricle; mb, moderator band; PA, pulmonary artery; RA, right atrium; RV, right ventricle. See Video 11.1.
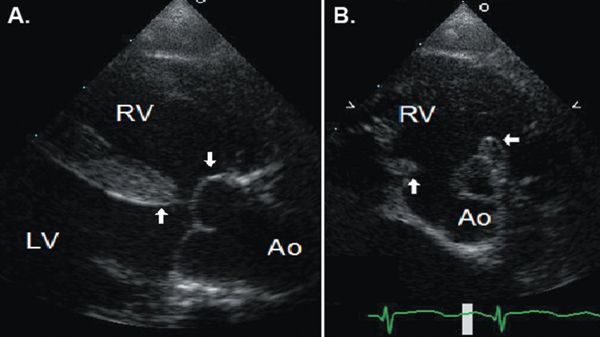
Figure 11.5. Anterior malalignment perimembranous ventricular septal defect in a patient with tetralogy of Fallot as seen in parasternal long-axis (A) and short-axis (B) views. Anterior deviation and rotation of the outlet septum results in the ventricular septal defect, an enlarged aortic outflow overriding the main body of the ventricular septum, and narrowed subpulmonary outflow. Ao, aorta; LV, left ventricle; RV, right ventricle. See Video 11.2.
A perimembranous VSD may be related to deficiency of tissue in the region of the membranous septum or may be due to malalignment of the outlet septum with the muscular ventricular septum. The gap caused by malalignment of the outlet septum can be due to either anterior or posterior deviation of the outlet septum. Anterior deviation creates a VSD of the type seen in tetralogy of Fallot (Fig. 11.5), with override of the aorta; this type of malalignment VSD can also be seen without associated pulmonary stenosis. Posterior deviation of the outlet septum creates a VSD of the type seen in association with interrupted aortic arch with muscular narrowing of the LV outflow tract (Fig. 11.6).
Because of their location adjacent to the tricuspid valve, perimembranous defects can be associated with tricuspid septal leaflet distortion and tricuspid regurgitation. Accessory tissue from the septal leaflet of the tricuspid valve, or a part of the septal leaflet itself, can partially or completely close the defect; this tissue is sometimes referred to as a ventricular septal aneurysm (Fig. 11.7). Occasionally, blood flow traverses through the VSD from the LV, through the aneurysmal tissue, and across the tricuspid valve and enters the right atrium resulting in LV–to–right atrial shunt (Fig. 11.8A, B). Misinterpretation of this high-velocity flow from this LV–to–right atrial shunt as tricuspid regurgitation can lead to an erroneous overestimation of RV pressure. A related, but distinct, type of LV–to–right atrial shunt is the Gerbode defect, which is an LV–to–right atrial connection created by a defect in the portion of the membranous ventricular septum separating the LV from the right atrium (Fig. 11.8C, D).
As perimembranous VSDs are also adjacent to the aortic valve, this valve can also be affected. In about 10% of perimembranous VSDs, there is associated aortic valve prolapse, and in 6% to 8%, there is associated aortic regurgitation. Aortic valve prolapse into the VSD is identified echocardiographically by identification of the right or noncoronary aortic cusp protruding into the VSD; this is typically best seen in the parasternal long- and short-axis views. Because aortic cusp prolapse is more common with subarterial defects, it is discussed in detail below. Because of its importance in the development of aortic regurgitation, the presence or absence of aortic cusp prolapse should be reported with any defect located immediately adjacent to the aortic valve.
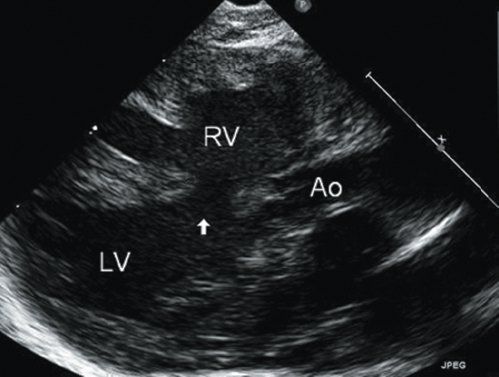
Figure 11.6. Posterior malalignment perimembranous ventricular septal defect in a patient with interrupted aortic arch. Posterior displacement of the outlet septum results in creation of the ventricular septal defect, narrowing of the left ventricular (LV) outflow tract, and enlargement of pulmonary outflow. Ao, aorta; LV, left ventricle; RV, right ventricle. See Video 11.3.
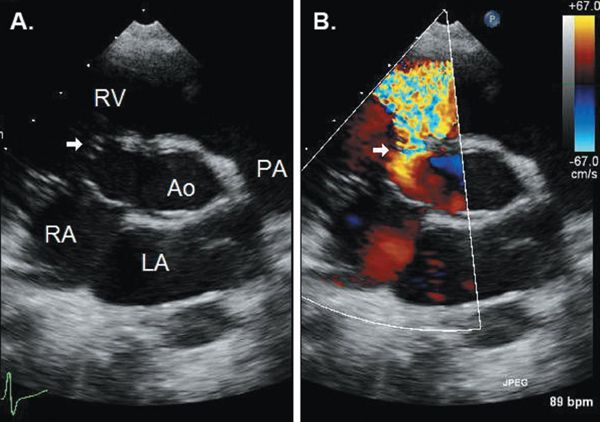
Figure 11.7. Ventricular septal aneurysm (arrow) made up of redundant tricuspid valve tissue that partially closes a perimembranous ventricular septal defect as seen in a parasternal short-axis view with two-dimensional and color Doppler. Ao, aorta; LA, left atrium; PA, pulmonary artery; RA, right atrium; RV, right ventricle. See Video 11.4.
A subaortic ridge, with or without significant subaortic obstruction, is seen in 3% to 6% of cases of perimembranous VSDs. In most of these cases, the subaortic ridge or ring is fibromuscular and is located immediately at the inferior aspect of the VSD (VSD is located distal to the ridge). In about half of these cases, there is an associated ventricular septal aneurysm. The subaortic ridge may develop with time; in a study by Eroglu et al., three-quarters of the patients developed a subaortic ridge after initial presentation. Blood flow disturbance beyond the subaortic ridge may also contribute to the development of aortic regurgitation.
Subarterial VSDs
Subarterial defects make up 5% to 10% of VSDs and are more common in the Asian population. These defects are located beneath both semilunar valves and result from a deficiency in the conal, or outlet, septum. Subarterial defects are seen by echocardiography to be immediately beneath the aortic valve in long-axis views and immediately adjacent to both the aortic and pulmonary valves in short-axis views (doubly-committed VSD) (Fig. 11.9). These defects are sometimes referred to as supracristal, but there is controversy regarding the definition of the crista supraventricularis, which determines the location of a supracristal defect. There is agreement that the crista supraventricularis is the muscle mass separating the tricuspid and pulmonary valves, but its exact location in relation to the parietal band, infundibulum, and septal band is inconsistently defined in the literature. As it is typically difficult to appreciate these muscle bands by echocardiography, we believe that the term subarterial is a more precise echocardiographic description. With subarterial VSDs, there is typically an absence of muscular tissue between the semilunar valves. However, occasionally the defect can be completely surrounded by muscle.
Prolapse of the right coronary cusp of the aortic valve into the defect, with distortion of the aortic valve, is present in up to 60% to 70% of subarterial VSDs. Prolapse is demonstrated as a diastolic bulging of the right aortic cusp and portion of the sinus into the RV in the long-axis and short-axis views (Fig. 11.9). It can be mild and transient during early systole, or severe, encompassing the entire cusp with tethering to the VSD throughout systole and diastole. The aortic cusp that is tethered into the defect will appear “beaked” and shortened in systole as compared to the uninvolved cusps. Because aortic prolapse can be associated with aortic regurgitation in up to one-third of patients, the presence or absence of aortic cusp prolapse should be reported with any defect located immediately adjacent to the aortic valve. Because of the risk of aortic regurgitation, some cardiologists advocate surgical repair of all subarterial defects regardless of the size of the VSD. However, development of and progression of substantial aortic regurgitation in patients with aortic cusp prolapse into a subarterial VSD is not universal. Cheung et al. reported that in patients with mild to moderate aortic cusp prolapse, 92% had no change or improvement in their degree of aortic regurgitation following VSD closure; none progressed to moderate or severe aortic regurgitation. In contrast, moderate to severe aortic cusp prolapse was associated with moderate to severe aortic regurgitation in most cases, and the degree of aortic regurgitation was unchanged or worsened following VSD closure with concomitant aortic valvuloplasty.
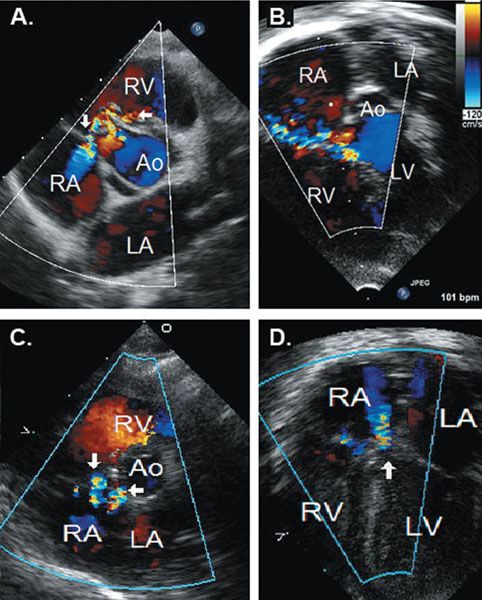
Figure 11.8. Occasionally, blood flow traverses through the VSD from the LV, through the aneurysmal tissue, and across the tricuspid valve and enters the right atrium resulting in LV–to–right atrial shunt (Fig. 11.8A, B). Misinterpretation of this high-velocity flow from this LV–to–right atrial shunt as tricuspid regurgitation can lead to an erroneous overestimation of RV pressure. A related, but distinct, type of LV–to–right atrial shunt is the Gerbode defect, which is an LV–to–right atrial connection created by a defect in the portion of the membranous ventricular septum separating the LV from the right atrium (Fig. 11.8C, D).
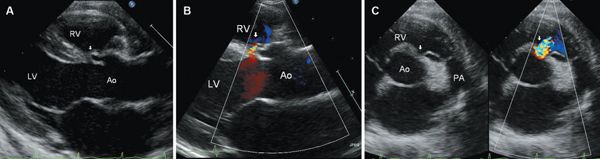
Figure 11.9. Parasternal long-axis (A and B) and parasternal short-axis (C) views of a subarterial ventricular septal defect (VSD) with prolapse of the right coronary cusp of the aortic valve (arrows) into the defect and mild aortic regurgitation. The subarterial defect is characterized by absence of muscular separation of the pulmonic valve from the VSD in the short-axis view. Ao, aorta; LA, left atrium; LV, left ventricle; PA, pulmonary artery; RV, right ventricle. See Video 11.6.
Inlet VSDs
Inlet VSDs are located posteriorly immediately adjacent to both AV valves. These defects are often best imaged from an apical four-chamber view or a parasternal short-axis view (Fig. 11.10). They most commonly occur as part of atrioventricular septal defects (AVSD) but can be isolated. Inlet VSDs should be distinguished from posterior muscular (also called inlet muscular) VSDs, which are located near the inlet septum but are separated from the AV valves by a rim of muscle. Inlet VSDs may be formed by malalignment of the atrial and ventricular septa, resulting in some degree of AV valve override and not infrequently with straddling of AV valve chordal attachments.
AV valve override occurs when an AV valve is positioned over a VSD and relates to both ventricles. AV valve straddling occurs when chordal attachments from an AV valve cross the VSD to the opposite side of the ventricular septum or contralateral ventricle. An AV valve can override, straddle, or both. Tricuspid valve straddling with chordal attachments to the left ventricular side of the septum can be seen with inlet VSDs (Fig. 11.11). The mitral valve typically does not straddle an inlet VSD but more commonly may straddle an outlet or a perimembranous defect. Because chordal apparatus crossing the defect impairs placement of the surgical patch, straddling and overriding of either AV valve must be accurately identified and interpreted by the echocardiographer.
Other types of AV valve involvement are common with inlet VSDs, most often involving the tricuspid valve. The tricuspid valve may be intrinsically abnormal with associated tricuspid regurgitation. The inlet defect may be partially or completely closed by redundant tricuspid septal leaflet tissue. When the VSD is associated with a partial AVSD, there is a cleft in the anterior leaflet of the mitral valve, which may result in regurgitation. Valve abnormalities associated with inlet VSDs should be identified as part of the complete echocardiographic evaluation.
Muscular VSDs
Muscular defects comprise 5% to 20% of VSDs. They can be described as being anterior, mid-muscular, posterior, or apical in location (Fig. 11.2). Anterior muscular defects are located anterior to the septal band (or trabeculum septomarginalis), which extends along the mid-septum from the insertion of the moderator band toward the membranous septum. The septal band bifurcates into an anterior and a posterior limb to surround the membranous septum and a portion of the outlet septum. The anterior muscular septum also includes anterior outlet defects, which are located in the smooth walled outlet septum but separated by a muscular rim from the semilunar valves. Mid-muscular defects are posterior to the septal band, anterior to the septal attachment of the tricuspid valve, and superior to the moderator band. Posterior muscular defects are located posterior to the septal attachment of the tricuspid valve; posterior-inlet muscular defects are located immediately below the AV valves but separated from the valves by muscle tissue.
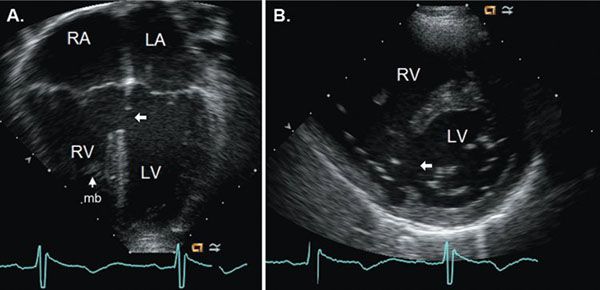
Figure 11.10. Inlet ventricular septal defect (arrows) imaged from apical four-chamber (A) and parasternal short-axis views (B). LA, left atrium; LV, left ventricle; mb, moderator band; RA, right atrium; RV, right ventricle. See Video 11.7.
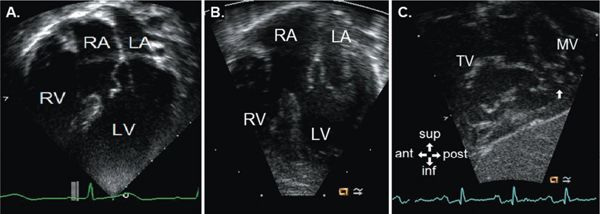
Figure 11.11. Apical four-chamber view (A) of the tricuspid valve overriding an inlet ventricular septal defect with tricuspid valve tissue partially closing the defect. Apical four-chamber (B) and subxiphoid oblique sagittal (C) en face views of a straddling tricuspid valve with attachments to the posteromedial papillary muscle of the left ventricle (LV) (arrow in C). ant, anterior; inf, inferior; LA, left atrium; MV, mitral valve; post, posterior; RA, right atrium; RV, right ventricle; sup, superior; TV, tricuspid valve. See Video 11.8.
Apical muscular VSDs are located inferior to the moderator band and include defects of the RV inflow apex (which is located more posteriorly) and the more anterior “infundibular” apex. The two RV apices are divided by dense trabeculations that run from the septum to the RV free wall; the LV may connect with the infundibular apex (43% of cases), the RV inflow apex (45% of cases), or both RV apices. In LV infundibular apical VSDs, there are usually multiple openings on the RV side of the septum. Hypertrophied RV apical trabeculations and the moderator band separate the infundibular apex and the VSD from the remainder of the RV. Occasionally, closure of these defects, either by surgery or transcatheter device, results in placement of the device or patch among RV trabeculae rather than across the true septum. This may result in closing off the infundibular apex of the RV, thereby making it physiologically part of the LV.
The best imaging plane for muscular VSDs is dependent on the exact location of the defect within the muscular septum; the ideal plane will demonstrate the VSD in the axial resolution and the flow across it parallel to the ultrasound beam. Determining the precise location of a muscular VSD may require combining information gained from several imaging planes as shown in the example in Figure 11.12. Defining the particular subtype of muscular VSD can be difficult because, with the exception of the moderator band, the muscular bands of the RV that are used to define the subtypes of muscular VSD can be difficult or impossible to visualize by echocardiography. To help clarify the location of a muscular VSD, we recommend describing the defect in both the anterior/posterior location as well as an apical/basal location. In the example shown in Figure 11.12, the defect is located in the RV infundibular apex anterior to the position of an imaginary apical extension of the septal band. Fig. 11.12 demonstrates a specific caveat of localization of VSDs using the apical four-chamber view. The apical four-chamber image plane demonstrates more posterior structures in the far field such as the atria and AV valves, but in the near field of the imaging plane it often displays a more anterior portion of the apical ventricular septum. The short-axis image in Figure 11.12 shows the more anterior location of this defect.
A patient may have multiple muscular VSDs, sometimes referred to as “Swiss cheese” septum, or may have a muscular VSD in addition to a VSD in another location (e.g., perimembranous or inlet). These additional defects can be easy to miss, especially when the first defect is large in size and there is equalization of pressures in both ventricles.
Special Considerations
Additional Abnormalities
As many as 60% of patients with VSDs have associated cardiac lesions. Some can be easily missed if not specifically looked for. Ventricular septal defects and aortic arch abnormalities keep close company. From 17% to 33% of patients with coarctation of the aorta have an associated VSD, as do nearly all patients with an interrupted aortic arch. The VSD is mid-muscular in almost half of these cases and perimembranous in almost a quarter of patients with coarctation and a VSD.
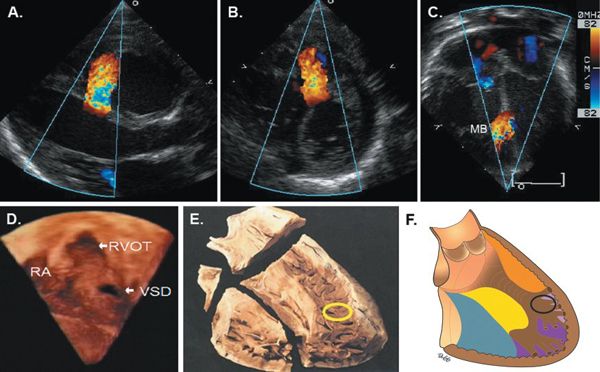
Figure 11.12. Localization of a muscular ventricular septal defect (VSD) near the right ventricular (RV) infundibular apex. Parasternal long-axis (A), short-axis (B), and apical four-chamber views (C) demonstrating localization of muscular VSD. D: Three-dimensional image acquired from a subxiphoid transducer position with the RV free wall cropped to show the entire septum from the RV aspect with localization of the defect in the anteroapical septum at the RV infundibular apex. Movie of this image demonstrates rotation of the septum with VSD imaged from left ventricular (LV) and RV aspects. E: Pathologic specimen showing the divisions of the ventricular septum as viewed from the RV aspect with the location of the muscular VSD circled. F: Representation of the subtypes of muscular VSDs with the location of the VSD seen in images A–D circled. MB, moderator band; RA, right atrium; RVOT, right ventricular outflow tract. See Video 11.9. (E, Reprinted and modified with permission from Becker A, Anderson R. Anomalies of the ventricles. In: Cardiac Pathology and Integrated Text and Colour Atlas. New York: Raven Press, 1983:12.2, Figure 12.1.)
A patent ductus arteriosus is commonly coincident with a VSD. With a large VSD and associated pulmonary artery systolic hypertension, near equalization of aortic and pulmonary artery pressure often results in laminar (and difficult to appreciate) ductal flow. This same ductus may be easy to appreciate after VSD closure, when pulmonary artery pressure is decreased.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


