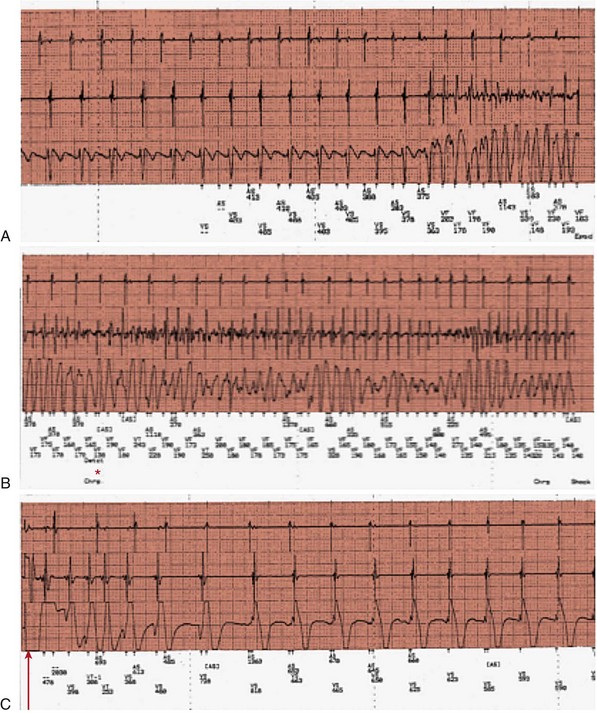
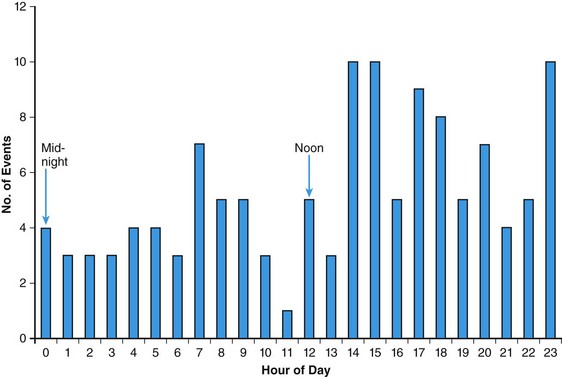
86 Hypertrophic cardiomyopathy (HCM) is a genetic cardiac disease with a heterogeneous clinical presentation.1–4 Although the clinical course is diverse and is compatible with normal longevity, unexpected sudden cardiac death (SCD) has been recognized as a devastating consequence of HCM5 since the initial description of this disease more than 50 years ago.1,2 Ventricular tachyarrhythmias have always been regarded as a prominent feature and a source of concern in this disease, primarily because of the perceived causal relationship between such arrhythmias and SCD.1–6 The present review represents a contemporary accounting of the profile of ventricular tachyarrhythmias, the current risk stratification algorithm and linkage with SCD, and, most important, the role the implantable cardioverter-defibrillator (ICD) in SCD prevention in HCM.7–9 Since the initial pathologic report of HCM in 1958,1 recognition that a small but critically important subgroup of patients with this complex disease are at increased risk for SCD has generated considerable attention and investigative work on the process of risk stratification, as well as evolving strategies for effective prevention of these largely unpredictable events.2,5,7,8 Although HCM is a complex and heterogeneous disease entity with diverse disease pathways, risk of SCD remains perhaps the most feared and devastating of its consequences, particularly in young patients (<30 years)1 (Figure 86-1). Figure 86-1 Top, Adverse clinical pathways in HCM. Each of these disease complications is associated with one or more potentially effective treatment options. AF, Atrial fibrillation; ICD, implantable cardioverter-defibrillator; RFA, radiofrequency ablation. Because of the observed association with SCD risk, identification of ventricular tachyarrhythmias has been regarded as a priority in HCM. However, on routine 24-hour ambulatory (Holter) electrocardiographic (ECG) monitoring,6 90% of HCM patients demonstrate ventricular arrhythmias (which are often frequent and complex), including premature ventricular depolarizations (≥200 in 24 hours in >20% of patients), ventricular couplets (>40% of patients), and, most important, bursts of nonsustained ventricular tachycardia (NSVT in 20% to 30% of patients).6 Furthermore, about 10% of HCM patients show particularly substantial ectopy, including (in combination) ≥200 premature ventricular depolarizations, ≥5 couplets, and ≥1 run of NSVT in a single 24-hour monitoring period (see Figure 86-1). Notably, this frequency of ventricular ectopy in HCM is disproportionate to the relatively low incidence of SCD in the overall patient population, representing a limitation to the reliability of ambulatory arrhythmias alone in predicting these events.1,2,5 Sustained monomorphic ventricular tachycardia (VT) is regarded as a high-risk marker in HCM,1,2,5,7,8 sometimes associated with left ventricular (LV) apical aneurysm.10 Furthermore, in an historical context, short and infrequent bursts of NSVT (usually 3 to 6 beats) were identified as markers of SCD in the early 1980s in two studies from tertiary HCM centers.1 Short bursts of NSVT identified on Holter ECG were linked to an eightfold enhanced risk for SCD (i.e., 8%/yr compared with <1%/yr in the absence of NSVT). Taken together, these initial clinical observations focused attention on the importance of ventricular tachyarrhythmias in HCM and stimulated considerable interest more than 30 years ago in ambulatory Holter monitoring as a means of stratifying the risk for SCD in such patients.1,2,5 Ventricular tachyarrhythmias identified on ambulatory ECG monitoring in HCM generally are clinically occult and are inconsistently associated with palpitations, light-headedness, or other cardiac events. However, greater clinical weight is instinctively given to NSVT when associated with such symptoms. Similar to other risk markers in HCM, NSVT is associated with high negative predictive value (i.e., 95%) and relatively low positive predictive value (i.e., about 20%),6 largely as a result of the low event rate and the clinical heterogeneity characteristic of HCM.5 Although specific evidence is lacking, prolonged isolated episodes of NSVT on ambulatory ECG (i.e., ≥10 beats) have been regarded, as a matter of clinical practice, to be markers of high risk in selected HCM patients. Despite its acknowledged limitations, screening by ambulatory (Holter) monitoring for NSVT has proved to be a useful and widely practiced noninvasive risk stratification strategy for determining SCD risk in adult HCM patients.1,2,5,6 For example, the absence of NSVT on 24- to 28-hour Holter (or longer-term) monitoring in HCM patients can support a low-risk clinical profile in conjunction with the absence of other risk factors, allowing patients a large measure of reassurance. However, the presence of NSVT on ambulatory monitoring may place patients in a potentially high-risk subgroup, for which further risk evaluation is justified with 21- to 30-day ECG monitoring to enable the clinician to better judge the arrhythmia profile over more extended periods. The pathogenesis of SCD in HCM is likely a complex and multifactorial process. Nevertheless, arrhythmia sequences documented by interrogation of stored ECG recordings from ICDs in high-risk HCM patients experiencing device interventions (Figure 86-2) offer a window to the mechanisms responsible for SCD, showing that the final common pathway for events is primarily ventricular tachyarrhythmias.2,4,8 Figure 86-2 Stored intracardiac ventricular electrogram from an asymptomatic 16-year-old boy implanted at age 15 for primary prevention of sudden death. This event occurred 10 months after implantation during a physical altercation. (A) After a period of sinus rhythm, ventricular fibrillation (VF) intervenes abruptly, (B) VF continues, and (C) defibrillator discharges appropriately with a 31-J shock, restoring sinus rhythm. Continuous recording is shown in three panels, with the tracing recorded from left to right. In a large, multicenter, and international ICD registry of high-risk HCM patients,7 appropriate device activations were triggered in each case by rapid VT or ventricular fibrillation (VF), thereby supporting the hypothesis that these primary arrhythmias are responsible for SCD events (see Figure 86-2). Bradyarrhythmias do not appear to play a major role in the pathogenesis of SCD in HCM, although bradycardia-mediated events cannot be absolutely excluded because of the backup pacing capability of the ICD. In one study, atrial fibrillation or sinus tachycardia was the initiating rhythm for sustained monomorphic VT in 56% of patients and in 84% of individual events, the latter suggesting that high sympathetic drive can be proarrhythmic in the presence of a susceptible substrate.11 Device activations in patients with HCM are not uncommonly triggered by rapid VT. Some investigators have suggested that when the ICD automatically intervenes for bursts of VT (within about 10 seconds after sensing a potentially lethal arrhythmia), the device is in effect only aborting arrhythmias that would have resolved spontaneously had the device not been present. However, such long runs of VT far exceed those evident on Holter recordings in this disease (in which runs of even 10 to 15 beats are regarded as highly unusual) and therefore are suggestive of increased risk.6 Consequently, it is generally regarded that episodes of VT that trigger the ICD in HCM are unlikely to be innocent in this disease, which is characterized by greatly increased LV mass and wall thickening, impaired LV compliance, and often outflow obstruction.1,2,5 In HCM, ventricular tachyarrhythmias probably emanate from the substrate of electrical instability and distorted electrophysiological transmission created by the disorganized left ventricular myocardial architecture1 and/or by bursts of myocardial ischemia (probably due to structurally abnormal and narrowed intramural arterioles), thereby leading to myocyte necrosis and repair in the form of replacement fibrosis and scarring1,5 (see Figure 86-1). This myocardial substrate may be vulnerable to a variety of internal triggers (related to the HCM disease process, such as abrupt increase in outflow obstruction) or to various extrinsic environmental factors such as intense physical exertion (i.e., in trained athletes). However, a component of individual susceptibility undoubtedly also plays a role in determining which HCM patients will experience life-threatening events at particular moments in the natural history of their disease. This is evident by the largely random circadian periodicity of appropriate ICD shocks from ventricular tachyarrhythmias throughout the day, triggered by a largely unpredictable electrophysiological substrate (Figure 86-3). These observations also suggest that home-based automatic external defibrillation (AED) strategies for SCD prevention are unlikely to be effective in HCM.12 Figure 86-3 Circadian distribution of appropriate ICD interventions provided over the 24-hour day for 126 VT/VF episodes in 63 HCM patients. (From Maron BJ, Semsarian C, Shen W-K, et al: Circadian patterns in the occurrence of malignant ventricular tachyarrhythmias triggering defibrillator interventions in patients with hypertrophic cardiomyopathy. Heart Rhythm 6:599-602, 2009.) Furthermore, particularly long-term survival (up to 30 years) following cardiac arrest, with or without ICD interventions, has been reported in HCM.13 In such patients, neither recurrent sudden death events nor progressive heart failure was inevitable or common, even after substantial periods of follow-up. Such observations underscore the unpredictability of the arrhythmogenic substrate in HCM, which may remain dormant over extended periods, thereby serving as a source of some reassurance concerning prognosis for HCM patients surviving profound arrhythmic events. Historically, in the pre-ICD era, management of high-risk HCM patients was limited to now obsolete prophylactic pharmacologic treatment strategies with β-blockers, verapamil, antiarrhythmic agents such as procainamide and quinidine, and later with amiodarone.1,2,5,14 However, to date, no studies support the efficacy of prophylactic drug treatment for SCD in HCM.2,14 One report, received 25 years ago from the UK but never updated, proposed that amiodarone was protective against the risk of SCD in symptomatic or mildly symptomatic HCM patients with NSVT on Holter ECG monitoring using a retrospective study design with only historical controls.2,14 Claims that amiodarone is absolutely protective against SCD in HCM no longer seem credible in light of more recent observations. For example, in studies in which the ICD is used in high-risk HCM patients,7,8 about 50% of patients experiencing an appropriate device discharge for VT/VF had been taking amiodarone concomitantly. Also, in a high-risk cohort in which only medications were used for arrhythmia protection, an important proportion had SCD (including >25% of those taking amiodarone).14 The possibility of severe pulmonary and other potentially toxic side effects associated with long-term administration of amiodarone also severely limits its application to SCD prevention in young HCM patients, who characteristically harbor long periods of risk over many decades.1,2,7 Prophylactic administration of β-blockers to young asymptomatic patients (sometimes in high doses) to reduce SCD risk is an obsolete strategy unsupported by evidence and is a remnant of the pre-ICD era.1,2 Since its introduction into clinical practice by Michel Mirowski more than 25 years ago, the ICD has achieved widespread acceptance as treatment for SCD prevention by automatically terminating life-threatening ventricular tachyarrhythmias and prolonging life, principally in high-risk patients with advanced coronary heart disease.15,16 In such patient populations, the superiority of the ICD over antiarrhythmic drug treatment has been documented in prospective, randomized trials of the ICD for secondary and primary prevention.15,16 However, not until 2000 was there an impetus for the systematic application of prophylactic device therapy to HCM patients.8 On reflection, Drs. Mirowski and Mower, when initially designing and promoting the ICD, did not consider or envision such device effectiveness in the context of the extraordinary pathology often encountered in HCM, with marked increases in LV mass1 and dynamic obstruction to LV outflow,1 microvascular ischemia,17 or diastolic dysfunction.1,2 Most data concerning the efficacy of the ICD in HCM18–22 were derived from a large multicenter international registry of patients considered to be at high risk for SCD based on the clinical judgment of their cardiologists and electrophysiologists.7,8 This prospectively and retrospectively studied cohort of 506 HCM patients was followed for an average period of 3.7 years after an ICD was implanted for primary or secondary prevention of sudden death. Appropriate device discharges (i.e., defibrillation shocks or antitachycardia pacing), triggered by VT/VF, occurred in 20% of patients, with an average discharge rate of 5.5%/yr. Furthermore, 45% of patients who received effective defibrillator therapy also experienced multiple appropriate interventions.
Ventricular Arrhythmias in Hypertrophic Cardiomyopathy
Historical Context
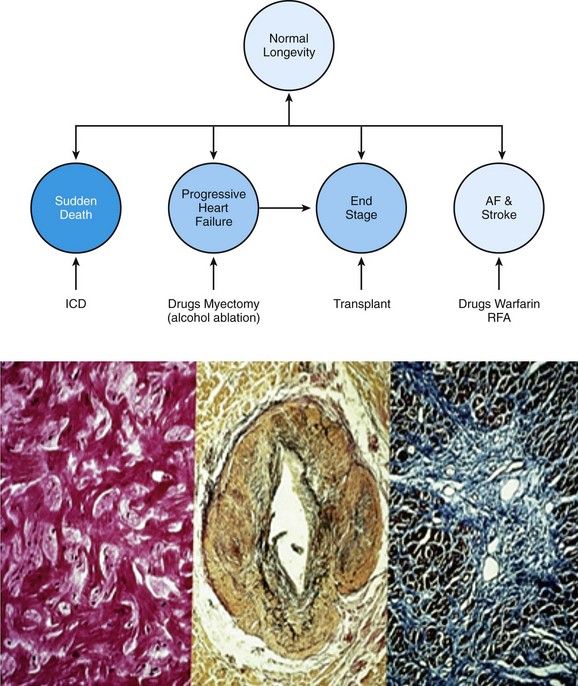
(From Maron BJ, Braunwald E: Evolution of hypertrophic cardiomyopathy to a contemporary treatable disease. Circulation 126:1640-1644, 2012; reproduced with permission of the American Heart Association.)
Bottom, Arrhythmogenic myocardial substrate in HCM. Left panel, Disorganized LV architecture with myocytes arranged at perpendicular and oblique angles. Center panel, Small-vessel disease; remodeled intramural coronary arteriole with thickened media and narrowed lumen. Right panel, Repair process with replacement fibrosis—the consequence of silent myocardial ischemia and myocyte death.
Ventricular Tachyarrhythmias
Mechanisms of Sudden Death
Prevention of Sudden Death
Pharmacologic Treatment
Implantable Defibrillator
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Ventricular Arrhythmias in Hypertrophic Cardiomyopathy
