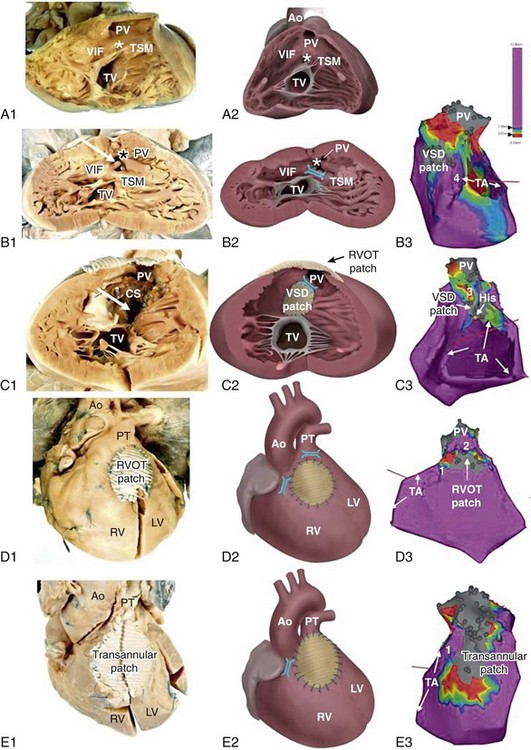
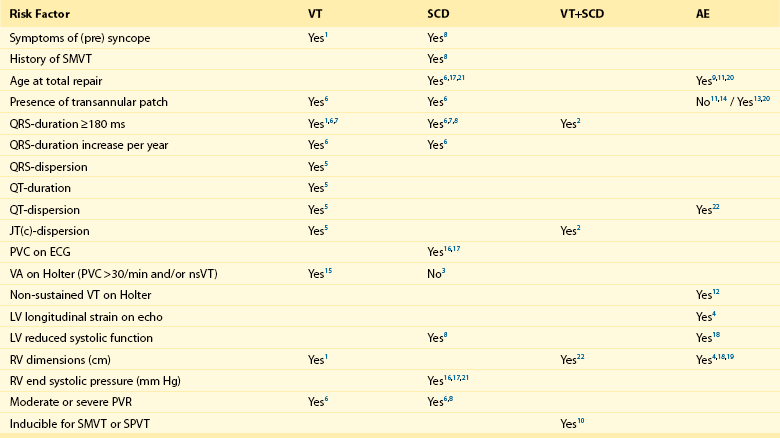
102 The reported prevalence of congenital heart disease (CHD) is 5.8 per 1000, with 11.9 per 1000 children and 4.1 per 1000 adults being affected. The prevalence of severe defects is 1.45 per 1000 children and 0.38 per 1000 adults. Of importance, between 1985 and 2000 the prevalence of severe CHD increased by 85% in adults but only by 22% in children.1 A clear change in mortality in CHD has been observed, with better survival in infancy and a trend towards death at older age.2 Improvements in survival are driven by a decreased mortality in moderate and severe forms of CHD in childhood, including tetralogy of Fallot (TOF), truncus arteriosus, atrioventricular septal defect, transposition of the great arteries (TGA), and univentricular hearts. This is likely the result of earlier surgical interventions and improved surgical techniques and outcomes. The increasing number of patients with repaired congenital heart disease joining the adult population requires the training of electrophysiologists with special interest in adult CHD as potentially life-threatening ventricular arrhythmias and sudden cardiac deaths (SCD) can still occur late after surgery. The incidence of SCD in repaired CHD is 0.9 to 1.4 per 1000 patient-years, which is 25- to 100-fold higher than for the general population.3,4 In one population-based series, 90% of all sudden deaths occurred in four main categories of CHD, being TGA, TOF, aortic stenosis and aortic coarctation with an apparent time-dependent incremental risk in patients with left heart obstructions and TOF. Six to nine percent of patients after repair of TOF died suddenly after 21 to 35 years of follow-up (2% to 3% per decade), accounting for up to 50% of all deaths in this group.3,5,6 The highest incidence of SCD was found in patients with aortic stenosis with SCD rates between 10% and 13% after 15 to 20 years of follow-up.3,7 The majority of CHD-related SCD is presumably due to ventricular arrhythmias, either hemodynamically not tolerated sustained monomorphic reentrant ventricular tachycardia (SMVT), with a higher prevalence in patients who have undergone ventricular incision and patch closure of ventral septal defect (VSD), or monomorphic and polymorphic VT and ventricular fibrillation (VF) in the absence of surgical scars. Although data are lacking, the latter arrhythmia mechanisms may be similar to those observed in other cardiac diseases with pathologic hypertrophy, fibrosis, impairment of cardiac function, and ultimately heart failure. Impairment of right and left ventricular function is likely to result in altered ion channel and transporter function. Downregulation of K+ currents and APD prolongation, which is a consistent finding in ventricular myocytes from subjects with cardiac dysfunction, promotes early afterdepolarization. In addition, changes in Ca2+ handling proteins, which are also observed in heart failure, can cause diastolic Ca2+ leak from the sarcoplasmic reticulum, resulting in delayed afterdepolarization and triggered activity. Advanced hypertrophy can be due to chronic pressure overload in left heart obstructions and unrepaired TOF. Ventricular dysfunction occurs if the right ventricle serves as the systemic ventricle after an atrial switch operation for TGA or in congenitally corrected transposition of the great arteries (ccTGA). Left ventricular dysfunction may be due to longstanding cyanosis if TOF repair is performed at an older age or if chronic volume overload after palliative shunting has occurred. Furthermore, long-lasting volume overload owing to chronic pulmonary regurgitation after initial correction contributes to ventricular dysfunction. Understanding of the different mechanism of ventricular arrhythmogenesis in CHD is crucial for both risk stratification and treatment. Current data on late morbidity and mortality are based on patients who underwent repair as adolescents. Early surgical intervention and changes in the surgical strategy in particular for TOF and TGA might not only influence early mortality but can also affect the incidence and the potential mechanism of arrhythmias and perhaps late morbidity and mortality in adult CHD patients in the future. Detailed descriptions of the most common forms of CHD related to ventricular arrhythmias are provided in the following sections. During normal development, the outlet portion of the heart needs to evolve from a single myocardial tube to a situation where the separated aorta and pulmonary trunk achieve their definitive positional relationship. This process requires proper septation of the outlet portion of the heart. Formation of the aortopulmonary septum (future outlet septum), orchestrated by neural crest cells, will result in separation of the common trunk into an aorta and pulmonary trunk. Asymmetrical, mainly subpulmonary myocardial contributions from the so-called second heart field will result in marked lengthening of the subpulmonary myocardium and will “push” the pulmonary trunk to its definitive position left anterior to the aorta.8 After proper development, the right ventricular outflow tract (RVOT) is characterized by the presence of a muscular subpulmonary infundibulum forming a circular muscular tube below the pulmonary valve. The posterior wall of the infundibulum, also known as the crista supraventricularis, is situated between the tricuspid valve and the pulmonary valve. The crista supraventricularis forms the summit of the ventriculo-infundibular fold (VIF), a fold of myocardium at the posterolateral wall of the RVOT, and extends toward the ventricular septum into the trabecula septomarginalis (Figure 102-1). The latter continues over the interventricular septum and contains the right bundle branch. The crista supraventricularis also encompasses the outlet septum (the muscular septum separating the aortic en pulmonary outlets), situated in between the ventriculo-infundibular fold and trabecula septomarginalis, which is small and not recognizable as a separate structure in the normal heart. In TOF, however, there is an anterior deviation of the outlet septum that, in contrast to normal, can be recognized as a separate structure, which is regarded as a pathognomonic feature of TOF (see Figure 102-1). The deviation of the outlet septum causes malalignment with the remainder of the ventricular septum, resulting in a subaortic, and in most cases perimembranous, ventricular septal defect. The deviation of the outlet septum also contributes to the subpulmonary stenosis. The amount of displacement and hypertrophy of the outlet septum determines the severity of the stenosis. The morphology of TOF encompasses a broad spectrum, with on the one end very slight malformations and cyanosis and on the other end severe forms of the disease with pulmonary atresia. Figure 102-1 Tetralogy of Fallot (TOF) isthmus. A, Anatomic specimen (A1) and drawing (A2) of the normal heart, right ventricular view. The heart is sectioned parallel to the interventricular septum and opened to allow a view into the right ventricle (RV). The fibrous tissues of the tricuspid valve (TV) and pulmonary valve (PV) are separated by the muscular tissues of the crista supraventricularis (CS), that consists of the continuum of the ventriculoinfundibular fold (VIF) and trabecula septomarginalis (TSM). The outlet septum (location indicated by the asterisk) is also part of the continuum, but cannot be identified as a separate structure in the normal heart. B, Anatomic specimen (B1) and drawing (B2) of a heart with unoperated TOF, same view as in A. The outlet septum (indicated by the asterisk), that in this case is small and fibrous, has deviated from the other components of the crista supraventricularis in an anterior direction, thus narrowing the ostium of the PV. The dextroposed aorta can be seen through the ventricular septal defect (arrow), which has a muscular rim (substrate for anatomic isthmus 4). B3, Electroanatomic voltage map (3-D mapping system, CARTOXP, Biosense Webster, USA) for a patient with operated TOF in a modified left posterior view. Voltages are color coded according to the color bar; grey tags indicate unexcitable tissue. Anatomical isthmus 4 is indicated. C, Anatomic specimen (C1) and drawing (C2) of operated TOF, same view as in A and B. The VSD patch drawn in place in C2 has been folded to the right in C1 to expose the VSD (arrow in C1). Note the hypertrophic crista supraventricularis (CS), at which site an infundibulectomy has been performed (C1). C3, Electroanatomic voltage map of a patient with operated TOF in a modified anterior view (same color coding). Anatomic isthmus 3 is indicated. D, Anatomic specimen (D1) and drawing (D2) of operated TOF, with a right ventricular outflow tract (RVOT) patch, frontal view. D3, Electroanatomic voltage map of a patient with operated TOF in a modified anterior view (same color coding). Anatomic isthmuses 1 and 2 are indicated. E, Anatomic specimen and (E1) and drawing (E2) of operated TOF, with a transannular patch, frontal view. E3, Electroanatomic voltage map of a patient with operated TOF in a modified anterior view (same color coding). Anatomic isthmus 1 is indicated. Ao, Aorta; PT, pulmonary trunk; PV, pulmonary valve; TA, tricuspid annulus. Total repair included (patch) closure of the perimembranous or muscular VSD and relief of the infundibular or valvular RVOT obstruction. This repair was initially performed through a vertical or transverse right ventriculotomy often combined with the use of an RVOT or a transannular patch to augment the restrictive RVOT or to relieve the stenosis of the pulmonary orifice. The malformation and type of repair are important determinants of potential reentrant tachycardia circuits, which is a common underlying mechanism of VT in repaired TOF.8 Areas of dense fibrosis owing to surgical incisions, but also patch material and the valve annuli, can form regions of conduction block that define reentry circuit borders and create intervening isthmuses of myocardial bundles that might contain the critical reentry circuit isthmus of a macroreentrant VT. Four anatomic isthmuses related to VT in repaired TOF have been identified9: isthmus 1 bordered by the tricuspid annulus and the scar or patch in the anterior RVOT, isthmus 2 between the pulmonary annulus and the RV free wall incision or RVOT patch sparing the pulmonary valve annulus, isthmus 3 between the pulmonary annulus and the VSD patch or septal scar, and isthmus 4 between the VSD patch or septal scar and the tricuspid annulus in patients with muscular VSDs (see Figure 102-1). The right ventriculotomy and the frequent use of a transannular patch with consecutive pulmonary regurgitation and chronic volume overload often resulted in RV dilatation and dysfunction, which is associated with VT and SCD in the long term (Table 102-1). Consequently, a combined transatrial-transpulmonary approach has been introduced. Currently, patch augmentation is avoided or usually limited to the pulmonary annulus whenever possible. This approach does not only positively affect RV function but can also prevent the anatomic isthmuses 1 and 2. Table 102-1 Risk Factors for Ventricular Tachycardia, Sudden Cardiac Death, and Adverse Events in Tetralogy of Fallot 1: Balaji et al.; Am J Cardiol; 1997 Jul 15;80(2):160-3. (n=135) 2: Berul et al.; J Cardiovasc Electrophysiol; 1997 Dec;8(12):1349-56. (n=101) 3: Cullen et al.; J Am Coll Cardiol; 1994 Apr;23(5):1151-5. (n=86) 4: Diller et al.; Circulation; 2012 May 22;125(20):2440-6. (n=413) 5: Gatzoulis et al.; Circulation; 1997 Jan 21;95(2):401-4. (n=99) 6: Gatzoulis et al.; Lancet; 2000 Sep 16;356(9234):975-81. (n=793) 7: Gatzoulis et al.; Circulation; 1995 Jul 15;92(2):231-7. (n=178) 8: Ghai et al.; J Am Coll Cardiol; 2002 Nov 6;40(9):1675-80. (n=125) 9: Karamlou et al.; Ann Thorac Surg; 2006 May;81(5):1786-93; discussion 1793. (n=249) 10: Khairy et al.; Circulation; 2004 Apr 27;109(16):1994-2000. (n=252) 11: Murphy et al.; N Engl J Med, 1993 Aug 26;329(9):593-9. (n=163) 12: Khairy et al.; Circulation; 2008 Jan 22;117(3):363-70. (n=68) 13: Nollert et al.; J Am Coll Cardiol; 1997 Nov 1;30(5):1374-83. (n=490) 14: Nollert et al.; Thorac Cardiovasc Surg; 1997 Aug;45(4):178-81. (n=71) 15: Harrison et al.; J Am Coll Cardiol, 1997 Nov 1;30(5):1368-73. (n=210) 16: Garson et al.; Circulation 1979 Jun;59(6):1232-40. (n=207) 17: Garson et al.; J Am Coll Cardiol; 1985 Jul;6(1):221-7. (n=488) 18: Knauth et al.; Heart; 2008 Feb;94(2):211-6. Epub 2006 Nov 29. (n=88) 19: Ortega et al.; Am J Cardiol; 2011 May 15;107(10):1535-40. (n=39) 20: Jonsson et al.; Scand J Thorac Cardiovasc Surg; 1995;29(2):43-51. (n=165) 21: Jonsson et al.; Scand J Thorac Cardiovasc Surg; 1995;29(3):131-9. (n=141) Myocardial histopathologic changes, in particular interstitial fibrosis of the muscular tissue of the crista supraventricularis, are more pronounced in patients who were operated at older age, specifically beyond the age of 4 years, and are thus subjected to long standing cyanosis (SaO2 < 80%) and higher end-diastolic RV pressure (>12 mm Hg).10 The clinical gold standard to detect ventricular fibrosis is late gadolinium enhancement (LGE) on cardiac magnetic resonance (CMR). Myocardial LGE was present in both the RV and LV of adults after repair of TOF and was related to increased age, and adverse clinical effects such as ventricular dysfunction and specifically RV LGE were associated with atrial and ventricular arrhythmias.11 Histopathologic changes can also contribute to the occurrence of complex ventricular ectopy, which has been considered a risk factor for fatal ventricular arrhythmias (VA). In particular, older age at repair has been associated with a higher grade of ventricular ectopy. Only 11% of patients in whom repair was performed between the ages of 4 and 15 years showed complex ectopy on Holter monitoring 6 to 12 months after operation, compared with 39.4% of patients who underwent surgery beyond the age of 15 years.10 The age dependency of the occurrence of complex ventricular ectopy could also be demonstrated in uncorrected patients. No significant ventricular ectopy (defined as Lown ≥ 2, <30 uniform PVCs/h) was observed in patients 0 to 7 years old. In contrast, 58% of patients 16 years or older had complex ectopy, with 21% having runs of nonsustained VT. Of interest in corrected patients, the relation of complex ventricular ectopy and time of repair persisted and was independent of the duration of follow-up or of the postoperative hemodynamic status.12 Although a higher grade of ectopy and nonsustained VT have been associated with VT inducibility, inconsistent data exist regarding their association with SCD. Accordingly, treatment of asymptomatic complex ventricular ectopy is not recommended.13 Primary repair in infancy before the age of 18 months is common practice since the early 90s and can be performed with low perioperative mortality. Avoiding long-standing prolonged hypoxemia and pressure overload can reduce the histopathologic changes and the substrate for slow conduction, complex ventricular ectopy, and fatal VA. Despite early operation, progressive pulmonary regurgitation occurs in almost all patients after transannular patch repair and is an important reason for reintervention. Although often tolerated, pulmonary regurgitation ultimately leads to RV dilatation and dysfunction, which can be further aggravated by residual RVOT obstruction. Moderate to severe PR and abnormal RV hemodynamics in particular, and increased RV end-systolic pressure have been associated with VT and SCD. In addition, a wide QRS (>180 ms) and an increase in QRS duration have consistently been reported as risk factor for SMVT and SCD.5 In particular, RV dilatation but not restrictive RV physiology has been associated with QRS prolongation, referred to as mechanoelectrical interaction.14 Impairment of RV function and LV hemodynamics have important roles. A moderate to severe left ventricular systolic dysfunction—defined as ejection fraction (EF) of less than 39% and 20%, respectively—was also more common in patients with TOF and (aborted) SCD.15 In addition, an left ventricular end diastolic pressure (LVEDP) ≥ 12 mm Hg was a strong and independent predictor of appropriate ICD shocks in patients with TOF who received an ICD for primary prevention.16 In selected patients, a reduction in QRS duration after pulmonary valve replacement (PVR) was related to a reduction in RV EDV assessed by CMR, which further supports the occurrence of mechanoelectrical interaction.17 A QRS duration greater than 180 ms 6 months after PVR, or no reduction of the QRS duration postoperatively, were strong predictors of adverse events defined as all-cause mortality, reoperation for pulmonary regurgitation, heart failure or VT.18
Ventricular Arrhythmias in Congenital Heart Disease
Ventricular Arrhythmias as Related to Specific Types of Congenital Heart Disease
Tetralogy of Fallot
Developmental and Anatomic Aspects
Type and Timing of Surgical Repair and Its Potential Relation to Risk Factors for Ventricular Arrhythmias
Effect of Pulmonary Valve Replacement and Intraoperative Cryoablation on Ventricular Arrhythmias
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Ventricular Arrhythmias in Congenital Heart Disease
