Chapter 65
Venous Tumors
Thomas C. Bower
Malignant venous tumors either originate primarily from the vein wall, extrinsically compressing or invading the vein, or grow within it as tumor thrombus.1–5 Most patients with these tumors have such advanced disease that operation cannot be offered. Surgical resection remains the mainstay of treatment, but there is a growing literature on the role of preoperative or postoperative adjuvant therapy.6–9 Secondary tumors of the superior vena cava (SVC) are rarely operable, so SVC obstruction is usually treated with venous stenting.2 Peripheral vein tumors may require concomitant arterial, bone, or adjacent soft tissue resection, with or without axial venous replacement.10–12 Because malignant neoplasms that affect the inferior vena cava (IVC) are most common,13–43 much of this chapter focuses on the diagnosis and treatment of them. The selection of patients, the surgical principles of management, and the techniques used to replace major veins are reviewed.
Definition and Tumor Types
Tumors of the IVC are classified by whether they involve the infrarenal, suprarenal, or suprahepatic segment. The suprarenal IVC has a retrohepatic portion behind the liver and an infrahepatic portion that is located between the caudate lobe and the renal veins.2,3
Intracaval tumor thrombus is defined by level or extent of IVC involvement. Level I thrombus extends to within 2 cm of the renal vein; level II thrombus extends into the suprarenal IVC but below the hepatic veins; level III thrombus is to the hepatic veins but below the diaphragm; and level IV thrombus extends into the right side of the heart.2
The types of primary and secondary tumors are shown in Box 65-1. Primary venous leiomyosarcomas (PVLs) occur more often than arterial sarcomas but are much rarer than retroperitoneal leiomyosarcomas.2 The first venous leiomyosarcoma was described by Perl in 1871.44 Suprarenal involvement occurs in more than 40% of cases,4 and three fourths of these tumors involve retroperitoneal and abdominal veins. The great saphenous vein is the most frequent site of PVL of the lower extremity.5
Venous leiomyosarcomas are polypoid or nodular, are firmly attached to the vein wall, and exhibit less intratumor hemorrhage or necrosis than other sarcomas.2,5 The most common growth pattern is intraluminal, but the tumor may grow through the vein wall and invade adjacent structures, which makes differentiation from other retroperitoneal sarcomas difficult.2,3 Distant metastases to the lung, liver, kidney, bone, pleura, or chest wall occur in half of the patients at the time of diagnosis,4,5 so survival is limited to months if surgery cannot be offered.4
Secondary cancers or sarcomas that involve the IVC are more common than PVL. Venous invasion, luminal obstruction by extrinsic compression, or intraluminal growth is the pathologic process (Fig. 65-1). Retroperitoneal sarcomas are the most common cause of malignant obstruction of the infrarenal segment but may affect higher levels.2,3 Sarcomas displace but rarely invade adjacent structures because of their pseudocapsule. However, the tissue planes between the tumor and IVC may be indistinct with large or irradiated tumors. Visceral or solid organ cancers affect the IVC in anatomic proximity to the site of origin of the neoplasm. For example, cancers of the liver, duodenum, pancreas, kidney, or adrenal glands involve the suprarenal IVC.2,3
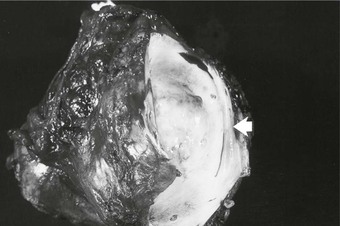
Figure 65-1 Pathology specimen showing both extraluminal and intraluminal growth of a retroperitoneal sarcoma. Invasion of the lumen is shown by the arrow.
A variety of cancers and sarcomas exhibit intraluminal tumor thrombus as part of their biologic behavior, with renal cell carcinoma (RCC) being most common (4%-15% of patients with this cancer).26–43 In fact, RCC is the most common cancer of the IVC that requires operative intervention.2 Tumor thrombus from RCC is found in the renal veins in 15% to 20% of cases21; the right kidney is affected more often than the left one, and tumors with thrombus tend to be larger than 4.5 cm in diameter.2,26,27 However, tumor thrombus can be seen with small renal cell or adrenocortical carcinomas (Fig. 65-2).2,3 In nearly half of patients with RCC and caval involvement, the tumor thrombus extends to within 2 cm of the renal vein–caval confluence (level I). Another 40% of patients have thrombus in the suprarenal IVC below the diaphragm (levels II and III), and in only 10% is thrombus in the right side of the heart (1% of all RCC patients).26,27 Similar to patients with PVL, survival of patients with secondary caval malignant neoplasms is measured in months without treatment.1–3,17
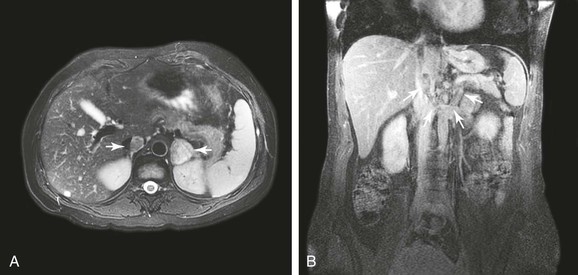
Figure 65-2 Axial (A) and coronal (B) images of a patient with a 3-cm adrenal tumor and associated inferior vena cava (IVC) tumor thrombus. The coronal image shows a 5-mm stalk of tumor thrombus extending from the left adrenal vein, through the left renal vein, and into the IVC (outlined by arrows).
Most extremity venous tumors occur secondary to sarcomas of the bone, cartilage, muscle, or fatty tissues.10–12 Malignant melanoma and fibrohistiocytoma may also cause peripheral vein compression or invasion. PVL of the superficial lower extremity veins occurs as a nodular mobile mass, whereas PVL of the deep veins often invades the adjacent soft tissues.5
Clinical Presentation and Evaluation
Primary leiomyosarcoma of the IVC is more common in women than in men, occurs over a wide age range, and has a mean patient age between 50 and 60 years.5,13–15 More than 80% of patients in the leiomyosarcoma registry compiled by Mingoli and colleagues were women.4 PVL of the peripheral veins affects men and women equally.5 Patients remain asymptomatic for a long time until symptoms or signs occur from metastatic disease or venous obstruction. Early detection is rare. Only 4 of 144 patients with IVC leiomyosarcoma in a review by Mingoli and colleagues had the tumor discovered incidentally.4 Abdominal pain is the most common presentation in 66% to 96% of patients.4,13 A palpable abdominal mass, lower limb edema, weight loss, Budd-Chiari syndrome, and vague symptoms (such as fever, weakness, anorexia, night sweats, and dyspnea) occur less often.4 Consumption coagulopathy is a rare but reported problem.2
Secondary caval tumors are detected in patients between the ages of 40 and 70 years.1–3,13–43 The mean age of patients who underwent IVC resection and replacement for malignant disease in one Mayo Clinic report was 52 years but ranged from 16 to 88 years.1 The majority of patients have symptoms and signs related to the cancer, not to IVC obstruction.1–3,13–25 IVC obstruction is a late sign, so vena cava involvement is identified on imaging studies. Symptoms and signs vary by the segment of IVC obstructed and include pain, arrhythmia, syncope, Budd-Chiari or nephrotic syndrome, and motor or sensory neuropathies. Lower extremity edema and deep venous thrombosis are rare problems with IVC tumors but are seen with primary tumors of the iliac or peripheral veins.1–3,5
A multidisciplinary team is critical to the evaluation and treatment of patients with venous tumors. Medical and surgical oncologists and subspecialists (vascular, hepatobiliary, urologic, orthopedic, neurologic, and cardiothoracic surgeons) are needed to direct the evaluation and to determine treatment. The goals of evaluation include the identification of the type and extent of tumor, a search for metastases, an assessment of the degree of venous obstruction, and the determination of patient risk and performance status.2,3
Computed tomography (CT), magnetic resonance imaging (MRI), ultrasonography, and rarely venography are used alone or in combination to accomplish these anatomic goals.1–3,7,17,18,45–50 CT and MRI are the most common tests used to define the location and extent of the tumor because the combination of axial, coronal, and sagittal images helps to plan the operation (Fig. 65-2). Venous phase imaging provides excellent views of the veins and collaterals. The role of venography is confined to the rare patient in whom histologic diagnosis by intraluminal fine-needle aspiration or true-cut needle biopsy would influence administration of preoperative adjuvant therapy2,3 or when computed tomographic venography or magnetic resonance venography inadequately defines venous occlusions and the collateral pathways.
MRI is the study of choice to define the upper extent of intracaval tumor thrombus and to differentiate bland from tumor thrombus in the infrarenal cava.43 Because not every patient can tolerate MRI, improvements in multidetector CT imaging have been welcomed. Guzzo and associates50 studied 41 patients after operation who had multidetector CT imaging before surgery. CT findings concurred with the intraoperative pathologic findings in 84% of this group, and the level of tumor thrombus was accurately depicted in 96% of patients. Few studies have compared MRI and CT in these circumstances. Two small studies show accuracies between 75% and 100% for CT and between 75% and 88% for MRI.48,49 MRI has been used to predict IVC wall invasion in patients with tumor thrombus. Those with an abnormal signal on either side of the caval wall on gadolinium imaging, an IVC diameter of 40 mm or more, a level III or level IV thrombus, or an IVC diameter of 18 mm together with a renal vein ostium diameter of 14 mm are at high risk of tumor adherence to the vein wall (90% sensitivity).46
Ultrasonography provides an accurate assessment of peripheral vein obstruction, but its ability to image the iliac system and IVC is hampered by bowel gas or when the tumor distorts the veins.2,3 Preoperative or intraoperative transesophageal echocardiography is sometimes used to corroborate the proximal extent of tumor thrombus if it is near the right atrium.26
If the tumor is localized and there are no metastases, a preoperative medical risk assessment is done, including a detailed cardiopulmonary evaluation. Of equal importance is an assessment of patient performance status.2 Patients in excellent physical condition with no or minimal limitation in daily activities (scores 0 and 1) have the best chance to maintain a similar quality of life after operation.1,17 Those who are either bedridden or need assistance in performing self-care (scores 3 and 4) are not offered operation at the author’s institution.
Treatment Approach and Type of Reconstructions
At the time of diagnosis, most patients with IVC tumors have advanced disease, which precludes operation. Those with diffuse metastases, poor cardiopulmonary function, or physical debility should not undergo surgical resection, in my opinion. In contrast, patients with localized tumors, a good performance status, and few medical comorbidities are candidates for surgical resection.1–3,13–25
Operative treatment and approach depends on the tumor type and its extent, the segment of IVC involved by the tumor, the degree of caval obstruction, and the status of collateral veins. The choice of incision is based on the patient’s body habitus, the segment of IVC affected, and whether major liver resection or cardiopulmonary bypass is needed. A midline abdominal incision works well to approach the infrarenal or infrahepatic segment for patients with narrow costal margins and for patients with enlarged subcutaneous abdominal wall venous collaterals (Fig. 65-3). A bilateral subcostal incision is useful for patients with wide costal margins who need infrahepatic or retrohepatic IVC replacement and concomitant liver resection (Fig. 65-4). This incision can be extended by a median sternotomy in patients with large RCCs and level III or level IV tumor thrombus in whom cardiopulmonary bypass may be needed. Last, some patients who need major liver resection and retrohepatic IVC replacement are best approached by a right thoracoabdominal incision through the eighth or ninth interspace.2
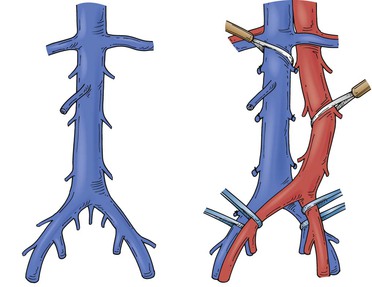
Figure 65-3 Tumors of the infrarenal segment can be approached through a midline abdominal incision. The infrarenal aorta and common iliac arteries often require partial mobilization to allow access to the lower vena cava and the proximal common iliac veins. Several lumbar veins may require division, and the surgeon must be wary of one to three small, middle, or lateral sacral veins that can cause troublesome bleeding if they are torn.
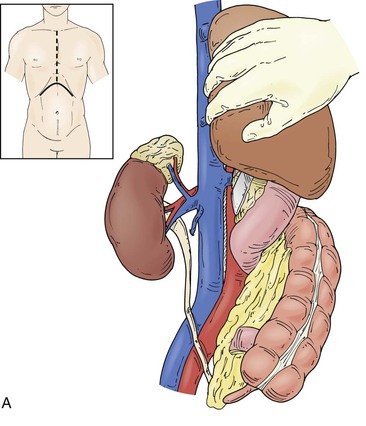
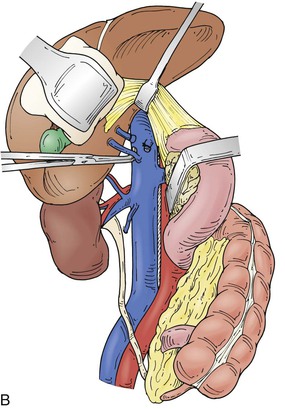
Figure 65-4 The suprarenal segment can be approached through a midline abdominal, bilateral subcostal, or a right thoracoabdominal incision. The abdominal incision can be extended through the sternum if access is needed to the right heart, as in some patients with tumor thrombus. A right medial visceral rotation is used to expose either the retrohepatic (A) or infrahepatic (B) segment. Further mobilization of the infrahepatic segment requires ligation and division of between one and four caudate lobe veins.
Surgical treatment of secondary tumors of the iliac and peripheral veins is also dependent on the extent of tumor, but adjacent arterial and nerve involvement must be anticipated.10–12 Long-segment chronic occlusions of the iliac veins rarely require replacement unless collateral veins are sacrificed in the course of resection. Axial lower extremity autogenous venous reconstruction is needed when preoperative imaging shows a paucity of collateral veins draining into the ipsilateral saphenous, deep femoral, or iliac systems. Contralateral saphenous vein is the first choice as a conduit and can be used as a straight, panel, or spiral graft. Venous reconstruction should follow arterial reconstruction when both vessels are resected. Anticipated patency rates of autogenous reconstructions in small clinical series have approached 80% at 2 and 5 years.12 A prosthetic graft has been used in the infrainguinal position but carries a lower patency rate than that of autogenous reconstructions.11 The precept I use for treatment of patients with lower extremity malignant neoplasms is that in-line autogenous or prosthetic reconstruction should be done in those who have patent axial veins at the time of tumor resection. This is particularly important for the common femoral or popliteal vein.
Inferior Vena Cava Resection without Replacement
The decision to replace the IVC during tumor resection is controversial but depends on whether the patient has problems from the caval obstruction, such as lower extremity edema or renal insufficiency.1–4,51 Patients with chronic IVC occlusion and well-developed venous collaterals that are not interrupted by operation can have the vena cava resected en bloc with the tumor with little venous morbidity.2 Patients with rapid occlusion of the IVC, few venous collaterals, and lower extremity edema are best treated with graft replacement.1–3
Although resection of the suprarenal IVC without replacement has been described, my preference always has been to reconstruct this segment because of the potential for acute kidney failure and lower extremity edema.1–3,18 The ability to predict which patients will tolerate resection without renal failure is difficult even if the paravertebral, lumbar, epigastric, adrenal, and gonadal venous pathways are patent.2,3 I reconstruct the remnant left or right renal vein if tumor resection involves a nephrectomy. Preservation of outflow through the remaining renal vein is necessary if the patient develops intraoperative anuria or an acute reduction in urine flow.1,16
Renal Cell Carcinoma with Inferior Vena Cava Tumor Thrombus
The most common malignant neoplasm to involve the IVC with intraluminal tumor thrombus is RCC. In most cases, except with very large cancers and bulky thrombus extending well into the retrohepatic vena cava, the thrombus can be removed en bloc with the cancer by transection of the renal vein at its confluence with the IVC.2,3,26,27 Early ligation of the renal artery may shrink the thrombus and “simplify” operation when the proximal extent of tumor is near the hepatic veins or right atrium.26,32 Most patients have tumor thrombus confined to the infrahepatic IVC. The tumor can be removed by clamping this segment after the caudate lobe veins of the liver have been divided,26 which causes little hemodynamic change (Fig. 65-4A). Patients with thrombus in the retrohepatic IVC extending to the hepatic veins may benefit from total vascular isolation of the liver to remove the tumor and to minimize blood loss. Dilated lumbar veins may cause troublesome backbleeding, even with caval clamping and inflow occlusion to the liver. Mobilization of the retrohepatic IVC on its right lateral side allows ligation of these rare veins. Another cause of backbleeding with total vascular isolation is a replaced left hepatic artery. Some patients with level III thrombus require venovenous bypass during total vascular isolation to support hemodynamics (total vascular isolation and venovenous bypass techniques are discussed under retrohepatic IVC replacement with major liver resection).26
Resection of a large RCC that involves the liver and has retrohepatic IVC tumor thrombus is challenging (Fig. 65-5). Choice of incision and exposure are key first steps. Nesbitt and colleagues suggest nephrectomy first, followed by ligation and amputation of the renal vein rather than en bloc resection.27 This maneuver facilitates access to and exposure of the vena cava and simplifies removal of the tumor thrombus. Renal artery embolization has been used to reduce tumor vascularity with large cancers and to shrink the tumor thrombus. However, few data support its routine use. The Cleveland Clinic group compared the results of 135 patients who had preoperative embolization, radical nephrectomy, and IVC tumor thrombectomy with those of 90 patients who did not have embolization.40 There was no benefit from this treatment. In fact, the authors found a higher mortality rate in the embolized group (13% vs 3%) and a fivefold higher risk of perioperative death by multivariable analysis. Moreover, such treated individuals had greater transfusion requirements and a higher postoperative complication rate (43% vs 29%). The level of tumor thrombus was not significantly reduced with this therapy. At the Mayo Clinic, renal artery embolization is reserved for palliation of symptomatic and inoperable RCC. Most blood loss during radical nephrectomy comes from disruption of dilated perirenal and retroperitoneal venous collaterals due to caval obstruction that are not decompressed with renal artery embolization. Early ligation of the renal artery, removal of the intracaval tumor thrombus, and restoration of normal IVC blood flow before the radical nephrectomy is completed helps decompress these veins.
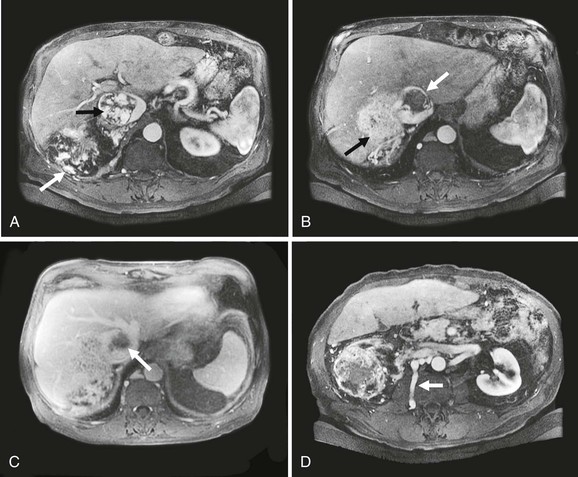
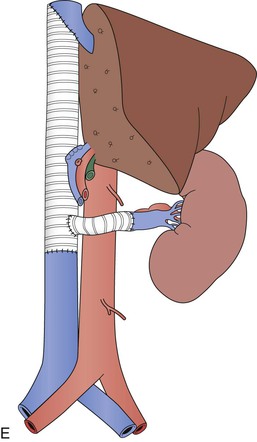
Figure 65-5 Axial CT images of a patient with a large vascular renal cell cancer, right liver lobe hepatic metastasis, and vascular thrombus extending both from the right hepatic vein and from the renal vein into the IVC, which is obstructed (A-C, arrows). Note the large lumbar vein collateral in D. The renal cell cancer was resected, the right lobe of the liver was removed, and because of caval wall invasion, the IVC was replaced from the suprahepatic segment to the infrarenal vena cava with a short graft to reconstruct the left renal vein (E). Note that the rings of the graft are kept intact at the anastomoses to avoid compression.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


