Chapter 52 Venous Thrombosis
Definitions
Deep vein thrombosis (DVT) refers to an obstruction of a deep vein by thrombus, usually in the legs or pelvis but occasionally in the arms. Thrombus from these sites can travel to the lungs to produce pulmonary embolism (PE), which occurs when the thrombus obstructs a pulmonary artery. This process is known as venous thromboembolism (VTE). Blockage of blood flow through the lungs and the resultant increased pressure in the right ventricle are responsible for the symptoms and signs of PE,1 whereas obstructed outflow of blood from occluded veins in the legs or arms produces symptoms and signs of DVT.
Epidemiology
Venous thromboembolism, which includes DVT and PE, represents the third most common cause of cardiovascular death after myocardial infarction (MI) and strokes. A first episode of VTE occurs in about 1 person per 1000 each year in the United States.2 The incidence rises exponentially with age, with 5 cases per 1000 persons per year by the age of 80 years. Although men and women are affected equally, the incidence is higher in Caucasians and African Americans than in Hispanic persons and Asian Pacific Islanders.3
Approximately one third of patients with symptomatic VTE present with PE; the remainder present with DVT alone.4 Up to half of patients with a first episode of VTE have no identifiable risk factors and are described as having unprovoked or idiopathic VTE. The remainder develop VTE secondary to well-recognized transient risk factors such as surgery or immobilization. Pulmonary embolism accounts for an estimated 15% of deaths in hospitalized patients, with at least 100,000 deaths from PE each year in the United States.
Pathobiology
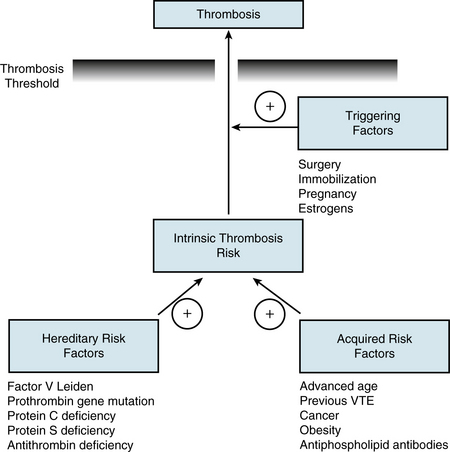
Pulmonary embolism and DVT are part of the spectrum of VTE and share the same genetic and acquired risk factors that determine the intrinsic risk of VTE for each individual5 (Fig. 52-1). Genetic risk factors include abnormalities associated with hypercoagulability of the blood, the most common of which are factor V Leiden and the prothrombin 20210 gene mutations. Acquired risk factors include advanced age, history of previous VTE, obesity, and active cancer, all of which limit mobility and may be associated with hypercoagulability. Superimposed on this background risk, VTE often occurs in the presence of a triggering factor that increases risk above the critical threshold. Such triggering factors (e.g., surgery, pregnancy, estrogen therapy) lead to vascular damage, stasis, and/or hypercoagulability, the components of Virchow’s triad.
In at least 90% of patients, PE originates from DVT in the lower limbs, and up to 70% of patients with proven PE still have demonstrable DVT upon presentation. Thrombi usually start in the calf veins.4 About 20% of these calf vein thrombi then extend into the popliteal and more proximal veins of the leg, from which they are more likely to embolize. Although often asymptomatic, PE can be detected in about 50% of patients with proximal DVT. Upper-extremity DVT involving the axillary and/or subclavian veins also can give rise to PE, but only 10% to 15% of such patients develop PE. Upper-extremity DVT most often occurs in patients with cancer, particularly those with indwelling central venous catheters. Unprovoked upper-extremity DVT, usually involving the dominant arm, can occur with strenuous effort—the so-called Paget-Schroetter’s syndrome.6
Clinical Manifestations
Recognizing VTE can be challenging because the symptoms and signs of both DVT and PE are neither sensitive nor specific for these disorders.1 A high index of suspicion is needed because DVT is often asymptomatic, and many cases of fatal PE go unsuspected prior to death. The clinical suspicion of VTE should be prompted by a constellation of risk factors, symptoms, signs, findings on electrocardiography, chest radiography, echocardiography, and blood test results. Although clinical assessment alone is insufficient to establish or exclude the diagnosis of VTE, clinical prediction rules are useful for establishing a pretest probability in which patients can either be classified as being at low, medium, or high probability of having DVT and/or PE, or as being unlikely or likely to have DVT and/or PE based on the estimated prevalence of the disease. Therefore, clinical pretest probability assessment serves as the root of algorithms for the diagnosis of DVT and PE.7
Diagnosis of Deep Vein Thrombosis
Because the clinical features are nonspecific, diagnosis of DVT requires objective testing. Patients who need such testing can be identified by their pretest likelihood of DVT, using validated clinical prediction rules (Table 52-1) that include components of the clinical assessment, presence of risk factors for VTE, and absence of an alternative diagnosis to explain the symptoms and signs.8 Based on the results of such an assessment, the pretest likelihood of VTE can be designated either as low, moderate, or high, or as unlikely or likely, and this assessment then guides subsequent selection of blood tests, such as the D-dimer assay, and noninvasive or invasive tests for definitive diagnosis of DVT. Tests for diagnosis of DVT also are relevant in patients with suspected PE because a diagnosis of proximal DVT in such patients provides sufficient grounds for initiation of treatment, and the treatment of DVT and PE are usually the same. Noninvasive tests include computed tomographic (CT) pulmonary angiography or ventilation/perfusion (V/Q) lung scanning for diagnosis of PE, and venous compression ultrasound for diagnosis of DVT. These tests have largely replaced pulmonary angiography (for the diagnosis of PE) and venography (for the diagnosis of DVT).
Table 52-1 Wells Clinical Prediction Model for Likelihood of Deep Vein Thrombosis
| Variable | Points |
|---|---|
| Predisposing Factors | |
| Active cancer | 1 |
| Paralysis, paresis, or recent cast immobilization | 1 |
| Bedridden more than 3 days or recent major surgery | 1 |
| Previous VTE | 1 |
| Symptoms | |
| Localized tenderness along deep veins | 1 |
| Swelling of entire leg | 1 |
| Signs | |
| Calf swelling over 3 cm compared with asymptomatic limb | 1 |
| Pitting edema | 1 |
| Collateral superficial veins | 1 |
| Clinical Judgment | |
| Alternative diagnosis at least as likely | − 2 |
| Clinical Probability | Total Points |
|---|---|
| Low | <1 |
| Intermediate | 1-2 |
| High | >2 |
| Unlikely | <2 |
| Likely | ≥2 |
VTE, venous thromboembolism.
Adapted from Scarvelis D, Wells PS: Diagnosis and treatment of deep-vein thrombosis. CMAJ 175:1087–1092, 2006.
Diagnostic Tests
D-dimer
A plasmin-derived degradation product of cross-linked fibrin, D-dimer can be measured in whole blood or plasma to provide an indirect index of ongoing activation of the coagulation system. Sensitivity of an elevated D-dimer level for the diagnosis of VTE ranges from 85% to 98%, but all available D-dimer assays have low specificities.9 False-positive D-dimer elevations can occur with advanced age, chronic inflammatory conditions, malignancy, and during the later stages of pregnancy and the postpartum period. In addition, hospitalized patients are more likely to have an elevated D-dimer level than outpatients, which renders the test less useful in this setting. Because of this lack of specificity, the value of the D-dimer assay resides with its high negative predictive value and the ability of a normal D-dimer to reduce the probability of VTE sufficiently to avoid further diagnostic testing in patients with a low or moderate pretest likelihood, who represent up to 30% of patients with suspected VTE.
Compression ultrasound
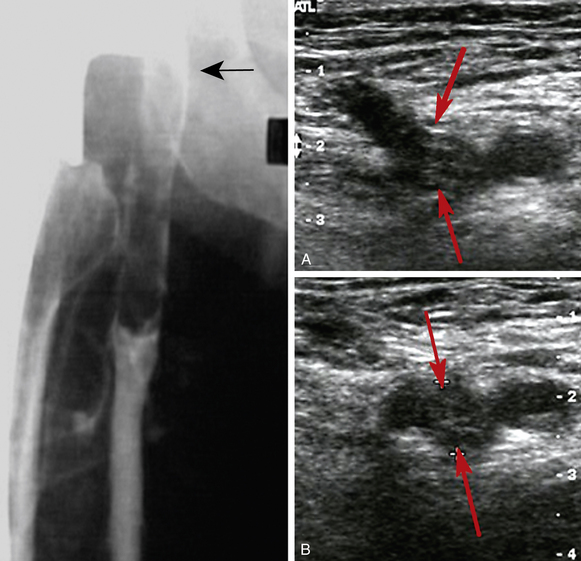
The diagnosis of DVT (also see Chapter 12) is usually based on venous compression ultrasound (Fig. 52-2), which has a sensitivity and specificity of about 95% for the diagnosis of proximal DVT.10 For calf DVT, the sensitivity of compression ultrasound is only about 73%. Because of the reduced sensitivity for detection of calf DVT, many centers restrict ultrasound testing to the proximal veins and only scan the region of the calf veins where they join the popliteal vein.
Computed tomographic venography
When combined with CT pulmonary angiography, CT venography is a simple test to diagnose DVT because both tests require only one injection of contrast dye. Compared with CT pulmonary angiography alone, the combination of CT pulmonary angiography plus CT venography increases sensitivity from 83% to 90%, but specificity remains unchanged, thereby resulting in only a modest increase in negative predictive value.8 Computed tomography venography adds significant radiation exposure and only marginally increases overall detection rate, so venous compression ultrasound is preferred because it provides the same information without exposing patients to ionizing radiation. Standard venography is not recommended in patients with suspected PE.
Contrast venography
Although contrast venography remains the gold standard for diagnosing DVT,11 the test is rarely performed in many centers (see Fig. 52-2). Venography is generally safe, but it can be uncomfortable and may be complicated by hypersensitivity reactions or superficial phlebitis. If the test is available, it should be considered when noninvasive testing is not diagnostic.
Diagnostic Strategies
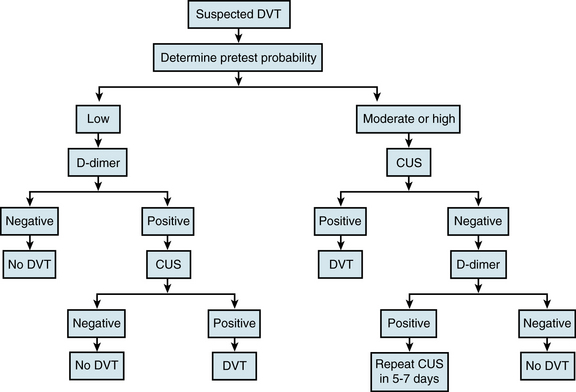
Diagnostic strategies usually start with assessment of the pretest probability of VTE, using validated risk assessment models for either DVT (see Table 52-1) or PE (see Chapter 14), depending on the clinical presentation.12 Patients can be classified as having a low, intermediate, or high pretest likelihood of VTE, or as being unlikely or likely to have VTE, and can then undergo further evaluation according to the appropriate algorithm.
Algorithm for diagnosis of deep vein thrombosis
Patients with a low pretest likelihood of DVT or those unlikely to have DVT should undergo D-dimer testing (Fig. 52-3). A negative D-dimer test excludes the diagnosis of DVT, whereas a positive test should prompt compression ultrasound examination.13 A negative ultrasound excludes the diagnosis, whereas a positive test establishes it. Patients with an intermediate or high pretest likelihood of DVT or those likely to have DVT should be sent directly for compression ultrasound examination. A positive compression ultrasound establishes the diagnosis. If the ultrasound is negative, it is helpful to then perform D-dimer testing. If the D-dimer test is negative, the diagnosis of DVT can be excluded. In contrast, a positive D-dimer test should prompt a follow-up compression ultrasound in 1 week to exclude the possibility of calf DVT with interval extension. Follow-up ultrasound testing in 1 week should be done routinely if D-dimer testing is not performed. The diagnosis of DVT can be excluded if the follow-up test is negative.
Whole-leg compression ultrasound examination can prevent the need for a follow-up examination in 1 week.14 This more time-consuming technique, which involves careful evaluation of the entire venous system, requires a high-performance scanner and an experienced operator. The advantage of this approach is that it will detect calf DVT as well as those in the more proximal veins. However, the accuracy of compression ultrasound for detection of calf DVT is lower than that for proximal DVT. Consequently, whole-leg ultrasound examination should only be performed in centers where the test has been validated.
Other tests
Thrombophilia testing has been advocated to identify patients with unprovoked (idiopathic) VTE who are at high risk of recurrence and who may therefore benefit from indefinite anticoagulation therapy. Although the common inherited thrombophilic disorders (see Fig. 52-1) are predictive of an increased risk of first-ever VTE, they do not appear to be associated with an increased risk of recurrence in patients who have already had an episode of VTE. Consequently, the results of thrombophilia testing rarely alter clinical management.15 Exceptions may include rare patients who are homozygous for factor V Leiden or prothrombin gene mutations, those with antithrombin, protein C, or protein S deficiency, patients with more than one thrombophilic disorder, and those with antiphospholipid antibody syndrome; such patients may benefit from long-term anticoagulation treatment (see Treatment).
Treatment
Although anticoagulant therapy remains the mainstay of treatment of VTE, patients with severe PE may require urgent reperfusion therapy to increase blood flow to the pulmonary arteries, thereby reducing pulmonary artery pressure. Therefore, rapid risk stratification is crucial to help guide treatment (see Chapter 14). The role of reperfusion therapy in patients with DVT is less clear. Venous thromboembolism patients who have contraindications to anticoagulant therapy may require insertion of an inferior vena cava filter.
Reperfusion Therapy
Patients with severe PE associated with hypotension or shock may benefit from pharmacological, mechanical, or surgical reperfusion therapy. Pharmacological reperfusion therapy involves systemic administration of a fibrinolytic agent. Mechanical reperfusion includes percutaneous catheter embolectomy with thrombus fragmentation, an approach that avoids the need for fibrinolytic drugs altogether,16 or catheter-directed fibrinolytic therapy, which requires lower doses of fibrinolytic agents than used for systemic administration. In some centers, surgical pulmonary embolectomy may be an option for patients who have severe PE and are at high risk for bleeding with systemic fibrinolytic therapy or who have failed such treatment.17 Mechanical techniques require skilled operators but provide useful alternatives to systemic fibrinolytic therapy for patients who have severe PE and are at high risk for bleeding.
The role of reperfusion therapy for DVT is less certain.18 Typically, such treatment is reserved for patients with extensive DVT involving the iliac and femoral veins. Systemic fibrinolytic therapy is rarely effective in this setting, so catheter-directed fibrinolysis or pharmacomechanical therapy is preferred. Pharmacomechanical therapy, which involves the use of clot maceration or extraction devices in conjunction with fibrinolytic therapy, not only produces more rapid lysis than that achieved with catheter-directed fibrinolytic therapy but is also associated with less bleeding because lower doses of fibrinolytic drugs are employed.
Although there is mounting evidence that reperfusion therapy results in higher rates of patency than are achieved with anticoagulant therapy alone, which lowers the risk of postthrombotic syndrome, definitive evidence of long-term benefit is lacking. Consequently, reperfusion therapy should only be considered for patients who are at low risk of bleeding and for those whose life expectancy is such that delayed benefits may be realized. For patients with extensive iliofemoral DVT or inferior vena cava thrombosis that is threatening the limb, reperfusion therapy may be the best option. In patients with less extensive DVT involving the iliac-femoral or femoral-popliteal veins, an ongoing clinical trial is assessing whether reperfusion therapy results in more rapid improvement in symptoms and reduces the risk of postthrombotic syndrome compared with anticoagulation therapy alone (www.clinicaltrials.gov: NCT00790335).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree