Chapter 43 Giant Cell Arteritis
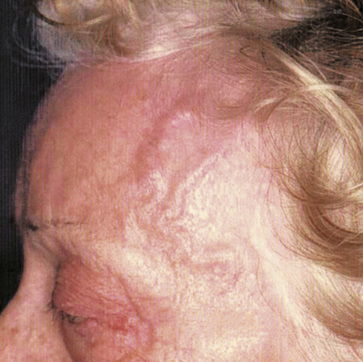
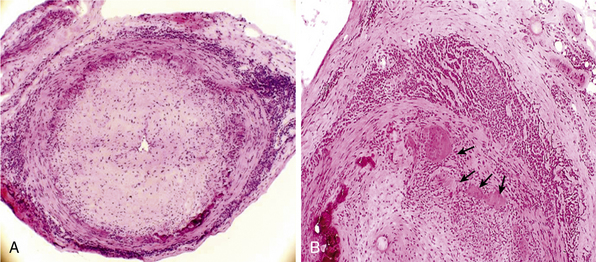
Giant-cell (temporal) arteritis (GCA) is a chronic inflammatory arteritis that preferentially involves large and medium-sized arteries and affects persons older than 50 years of age. Although autopsy studies have shown that the aorta and its major tributaries are almost invariably involved, most of the major clinical manifestations and complications of the disease arise from involvement of the carotid artery branches and include headaches, visual loss, and stroke.1–3 Aneurysms due to aortitis, as well as extremity arterial occlusions and claudication, can also be seen in GCA, especially in late disease.4,5 Histopathological diagnosis is usually determined from examination of a biopsy of the superficial temporal artery (Figs. 43-1 and 43-2).
Some 50% of patients with GCA develop polymyalgia rheumatica (PMR), a clinically defined syndrome consisting of aching and stiffness in the neck, shoulders, or pelvic girdle. Polymyalgia rheumatica can also exist as a distinct entity with no evidence of vascular involvement.6
Epidemiology
Giant-cell arteritis characteristically occurs in people older than 50 years of age, and its frequency increases with age. Maximal incidence of GCA occurs in persons between 75 and 85, and it is twice as frequent in women as men. Although there is a markedly increased incidence of GCA among Caucasians in northern Europe and in populations with similar ethnic background,7 the disease can occur in all populations. Giant-cell arteritis is not an uncommon disease. Studies conducted in northern Europe and North America disclose an annual incidence of 19 to 32 cases per 100,000 people older than 508,9 and a rate of 49 per 100,000 for individuals in their 80s.7,9 In Mediterranean countries, the annual incidence is lower, about 6 to 10 cases per 100,000.10,11 Isolated PMR is even more frequent, with an average annual incidence of 52 per 100,000 among people older than age 50.6,7
Pathology
In the large and medium-sized arteries involved in GCA, the vessel wall is infiltrated by T lymphocytes and macrophages, frequently forming a granulomatous reaction with the presence of multinucleated giant cells12 (see Fig. 43-2). Scattered polymorphonuclear leukocytes can be occasionally identified. B lymphocytes are scarce and natural killer (NK) cells are virtually absent.13
Inflammatory infiltrates usually reach the artery through the adventitial layer and subsequently expand to involve the entire vessel thickness. Extent of inflammatory infiltrates is highly variable among patients and even varies in different sections of the same specimen. The internal elastic lamina is usually disrupted, and giant cells often accumulate in its vicinity but are not required for the diagnosis. In well-developed lesions, the lumen is occluded by intimal hyperplasia. In some specimens, inflammation of the vasa vasorum and small vessels surrounding the temporal artery is the only apparent abnormality.14 When inflammation of small branches is the only abnormality, other forms of systemic small- and medium-vessel vasculitis must be excluded.
Inflammatory lesions are typically segmental and preferentially distributed along the carotid and vertebral arteries. Intracranial vessels are usually spared. Small arteries and arterioles in the vicinity of the superficial temporal artery are almost invariably involved.14 Autopsy studies have demonstrated that inflammatory infiltrates are often detected in the aorta and its major tributaries.15
Histopathological findings in PMR include chronic synovitis and bursitis of proximal joints.16,17 Synovitis is usually mild, and inflammatory infiltrates contain CD4 T lymphocytes and macrophages, with scarce granulocytes. Muscle biopsies do not disclose specific abnormalities.
Pathogenesis
Genetic Predisposition
The predominance of GCA and PMR among Caucasians, especially those of northern European origin, strongly suggests a genetic component in the pathogenesis of GCA and PMR. A higher prevalence of HLA class II alleles DRB1*04 has been reported among patients with GCA and PMR compared to the general population.18 Certain polymorphisms of genes involved in immune and inflammatory responses, such as tumor necrosis factor (TNF)-α, vascular endothelial growth factor (VEGF),19,20 endothelial nitric oxide synthase (eNOS),21 intercellular adhesion molecule (ICAM)-1,22 interleukin (IL)-6, and mannose-binding lectin23 among others,24 appear to be more frequent in patients with GCA than in the general population. The influence of these polymorphisms in the development of GCA remains undefined but is under active investigation.
Possible Triggering Agents
Several observations suggest that GCA may be caused by an environmental triggering agent yet to be defined. Cyclic fluctuations in the incidence of GCA occurring every 6 or 7 years have been reported.9 Additionally, the characteristic granulomatous reaction with multinucleated giant cells has also prompted a search for an infectious agent that may cause a delayed-type hypersensitivity reaction. However, attempts to identify specific pathogens, including Chlamydia pneumoniae and parvovirus B19, have led to conflicting results,25,26 and the search for an infectious etiology of GCA continues.27
Immunopathogenic Mechanisms
It has been postulated that inflammatory lesions in GCA develop as a consequence of an antigen-specific immune response against antigens present in the vessel wall. Activated dendritic cells have been detected in inflammatory lesions of GCA,13,28 where they are thought to serve as antigen-presenting cells and provide co-stimulatory signals for CD4 T-cell activation. Innate immune system activation through Toll-like receptors (TLRs) seems to be relevant in the activation of dendritic cells.29 Immunopathological studies have demonstrated that inflammatory infiltrates in GCA mainly consist of activated macrophages and T lymphocytes, particularly of the CD4 subset.13 Expansion of CD4 T-lymphocyte clones derived from different areas of temporal artery biopsy specimens has shown that some of them share identical sequences at the third complementary determining region of the T-cell receptor,30 suggesting a specific immune recognition of a disease-relevant antigen. CD4 T lymphocytes undergo T helper 1 (TH1) differentiation, with vigorous production of interferon (IFN)-γ, a key cytokine in macrophage activation and granuloma formation.31 Interleukin-17 is also produced in GCA lesions, providing evidence for the participation of TH17-mediated responses in the pathophysiology of GCA.32
Mechanisms of Vascular Injury
Multiple processes have been implicated in the vascular injury seen in GCA. Activated macrophages produce oxygen radicals and proteolytic enzymes that participate in disruption of the vessel wall and tissue destruction.33,34 Increased levels of lipid peroxidation products, aldose reductase, inducible nitric oxide synthase (iNOS), and functionally active matrix metalloproteases MMP-2 and MMP-9 have been demonstrated in GCA.33,35
Systemic Inflammatory Response
Activated macrophages produce proinflammatory cytokines IL-1, TNF-α, and IL-6, which are major inducers of the systemic inflammatory response (fever, weight loss, anemia, and hepatic synthesis of acute-phase proteins) often prominent in patients with GCA. Expression of these cytokines in the arterial tissue of patients, as well as circulating levels of TNF-α and IL-6, correlate with the intensity of the systemic inflammatory reaction.36 As discussed later, these cytokines are able to activate proinflammatory cascades such as chemokine release, adhesion molecule expression, and angiogenesis. Although each of these cascades may amplify and maintain the inflammatory process,37 some of their effects on vessel wall components might be protective against vascular occlusion.
Vascular Response to Inflammation
Vessel wall components, particularly endothelial cells (ECs) and smooth muscle cells (SMCs), actively react to cytokines and growth factors released by infiltrating leukocytes. Vascular response to inflammation leads to amplification and perpetuation of the inflammatory process and vessel occlusion.37 Inflammation-induced angiogenesis is remarkable in GCA lesions and preferentially occurs at the adventitial layer and within inflammatory infiltrates, particularly in granulomatous areas at the intima media junction.38,39
Neovessels may have a protective role at distal sites where providing new blood supply may prevent organ ischemia.39 Additionally, neovessels provide a population of ECs that may exert a variety of proinflammatory activities.38 Vascular response to inflammation may eventually lead to vessel occlusion, with subsequent ischemia of the tissues supplied by involved vessels. In GCA, vessel occlusion results mostly from intimal hyperplasia driven by proliferation and matrix production by myointimal cells; platelet-derived growth factor (PDGF) and transforming growth factor (TGF)-β appear to be crucial cytokines in this process.37
Clinical Manifestations
Patients with GCA may present with a wide variety of clinical features, summarized in Table 43-1. Disease-related manifestations may appear rapidly or develop insidiously. A delay of weeks or even months between the onset of clinical symptoms and diagnosis is common, particularly in patients with predominantly systemic complaints in whom more prevalent diseases (e.g., infections, malignancies) are usually suspected. Ischemic complications tend to accumulate in some patients and are frequently early events during the course of the disease.40,41
Table 43-1 Clinical Findings in a Series of 250 Patients with Giant Cell Arteritis
| General Features | |
| Age (mean, range) | 75 (50-94) |
| Sex (female/male) | 178/72 |
| Weight loss | 61% |
| Fever | 47% |
| Cranial Symptoms | 86% |
| Headache | 77% |
| Temporal artery abnormality (swollen, tender, weak/absent pulse) | 74% |
| Jaw claudication | 44% |
| Scalp tenderness | 39% |
| Facial pain | 18% |
| Earache | 18% |
| Odynophagia | 12% |
| Ocular pain | 8% |
| Tongue pain | 5% |
| Carotodynia | 5% |
| Toothache | 5% |
| Trismus | 1% |
| Ophthalmic Events | 22% |
| Blindness (permanent) | 14% |
| Amaurosis fugax | 10% |
| Transient diplopia | 4% |
| Cerebrovascular Accident | 2% |
| Symptomatic Large Vessel Involvement (Claudication and/or Bruit) | 5% |
| Polymyalgia Rheumatica (PMR) | 48% |
Modified and reprinted from Cid MC , Hernandez-Rodriquez J, Grau JM: Vascular manifestations in giant-cell arteritis. In Asherson RA, Cervera R, editors: Vascular manifestations of systemic autoimmune diseases, London, 2001, CRC Press.
Some data suggest that an initial presentation of GCA that includes signs and symptoms of systemic inflammation (elevated acute phase reactants, fever, anemia, weight loss) is associated with a specific pattern of clinical illness. For unclear reasons, patients with a strong systemic inflammatory response are at lower risk of ischemic events but are more refractory to therapy40,42 than patients with a seemingly weaker initial inflammatory response. Similarly, as with cranial ischemic complications, symptomatic stenosis of large vessels has been shown to be negatively associated with prominent inflammatory markers at the time of diagnosis.43
Systemic Manifestations
Patients with GCA or PMR frequently experience malaise, anorexia, weight loss, and depression.1,3,44 Nearly 50% of patients with GCA have fever, and in some patients, fever or constitutional symptoms are the most prominent findings. Approximately 10% of patients present with fever of unknown origin with subtle or no cranial manifestations.6
Clinical Manifestations of Cranial Arterial Involvement
Headache is one of the most common and classic symptoms and occurs in 60% to 98% of cases of GCA.6 Intensity and location of headache are highly variable, but it frequently predominates at the temporal areas. Scalp tenderness is also common. About 40% of patients experience jaw claudication when eating, a symptom highly specific for GCA but occasionally seen in other vascular diseases (e.g., necrotizing vasculitis, systemic amyloidosis). Other manifestations, such as facial swelling, tongue ischemia, or edema, are less frequently seen6,45 (see Table 43-1). Patients may also present with a variety of unusual pains in the craniofacial area, including ocular pain, earache, toothache, odynophagia, odontalgia, and carotidynia. When certain of these symptoms predominate, a substantial delay in diagnosis is common.
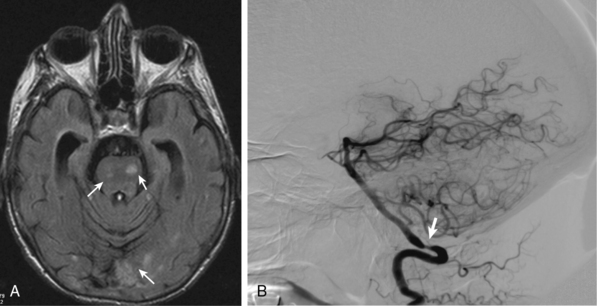
Less common ischemic complications include stroke due to involvement of carotid or vertebral tributaries, and scalp or tongue necrosis. Strokes, when they do occur, are more frequent in territories supplied by the vertebral arteries (Fig. 43-3). Additional ischemic manifestations include hearing loss and vestibular dysfunction.46
Cardiac, Aortic, and Peripheral Vascular Manifestations
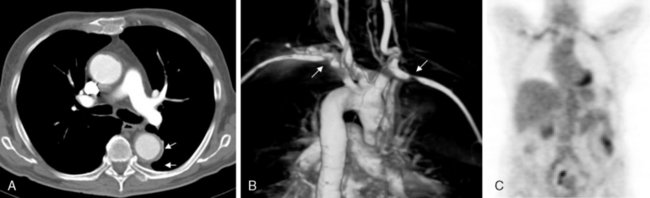
Aortitis and inflammatory involvement of the main branches of the aorta are more common in GCA than generally appreciated (Fig. 43-4). In a small autopsy series, aortic inflammation was observed in 12 out of 13 patients with GCA. Imaging techniques are now used to indirectly measure aortic inflammation in living individuals. Evidence of probable aortic involvement can be detected by positron emission tomography (PET) or computed tomographic angiography (CTA) in 50% to 65% of patients at the time of diagnosis47,48 (see Fig. 43-4). Aortic involvement is asymptomatic unless aortic dissection or dilation occurs (Fig. 43-5). Systematic screening of 54 patients revealed that aortic dilatation occurs in 22.5% of patients after a median follow-up of 5.6 years. Interestingly, unlike noninflammatory aortic disease, there is a markedly increased ratio of thoracic to abdominal aortic aneurysms in patients with GCA, and the risk of thoracic disease may be 17-fold higher among patients with a history of GCA.49
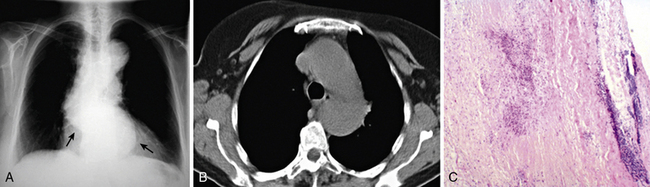
Figure 43-5 Aortic aneurysm in patient with giant cell arteritis (GCA) discovered 5 years after diagnosis.
Giant cell arteritis may also involve aortic branches (see Fig. 43-4). Positron emission tomography scan studies show that subclavian arteries may be involved in 70% of patients, axillary arteries in 40%, iliac arteries in 37%, and femoral arteries in 37%. Studies using color duplex ultrasonography (US) have disclosed abnormalities in these territories in about one third of patients.50 Distal lower-limb arteries (e.g., femoropopliteal, tibial, peroneal) may also be affected.5 Although in most instances, extremity involvement is asymptomatic, some patients develop claudication and even critical ischemia when significant vascular stenoses occur. Large-vessel occlusions are usually late manifestations of GCA, often occurring years after initial diagnosis. When they become clinically significant, they often are misdiagnosed as secondary to atherosclerotic disease. For this reason, the frequency of clinically relevant peripheral artery disease in GCA is not well known.
Myocardial or mesenteric infarction due to GCA is seen infrequently.51 Because GCA occurs in older people, some cases of inflammatory coronary or mesenteric arteritis may be misdiagnosed as due to atherosclerotic disease. Even among patients with GCA, however, atherosclerosis is a much more common cause of cardiac or mesenteric ischemia than vasculitis. With increased interest in the role of inflammation in coronary artery disease (CAD) and new tools to study the disease process, investigators are now better able to study the contributions, if any, of inflammatory arteritis to CAD among patients with GCA and other vasculitides.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree