Chapter 47 Venous Thromboembolic Disease
Pathophysiology of Venous Thrombosis
Venous thrombosis is governed by the three principles of Virchow triad: hypercoagulability, stasis, and endothelial injury. Activation of coagulation appears to be critical in the pathogenesis of DVT. Although the hemostatic system is continuously active, thrombus formation is ordinarily confined to sites of local injury by a precise balance between activators and inhibitors of coagulation and fibrinolysis. A prothrombotic state may result either from imbalances in the regulatory and inhibitory systems or from activation exceeding antithrombotic capacity.1 Regardless of etiology, most venous thrombi originate in areas of static, low blood flow, often behind valve pockets.2 Furthermore, many risk factors for acute DVT are associated with immobilization and slow venous flow, and several mechanisms have been advanced to explain the role of stasis in thrombogenesis. For example, in comparison to pulsatile flow, static streamline flow is associated with profound hypoxia at the depths of the venous valve cusps and may induce endothelial injury.3 Under basal conditions, the endothelium provides a vasodilatory and local fibrinolytic environment. In this setting, coagulation, platelet adhesion, platelet activation, inflammation, and leukocyte activation are inhibited.4 After a disturbance of the endothelium, a prothrombotic and proinflammatory state occurs and is defined by vasoconstriction, activated platelets, and upregulation of cellular mediators to recruit inflammatory cells to the site of endothelial damage.4 Individually, stasis, endothelial injury, and activated coagulation factors may be insufficient to provoke thrombosis.5 However, when localized in regions of stasis, and incited by endothelial disruption, the coagulation cascade allows activated factors to rapidly intensify the thrombotic stimulus leading to platelet aggregation, coagulation factor localization, and fibrin formation.5,6 Thus, thrombosis appears to be a multifactorial phenomenon, with convergence of several pathologic factors often required to produce a thrombotic event.7
The pathophysiology of venous thrombosis is a cyclical event, amplified by the associated inflammatory process.8 With inflammation, there is an increase in tissue factor, platelet reactivity, fibrinogen, phosphatidylserine expression on membrane surfaces, and PAI-1 (inhibiting fibrinolysis) and a decrease in thrombomodulin (and thus a decrease in the activity of protein C).9 Cell adhesion molecules allow leukocyte transmigration, and selectins are intimately involved in this process. Venous stasis and ischemia result in upregulation of P-selectin, and this localizes microparticles, which are prothrombotic, to the area of evolving thrombosis.10,11 The role of the inflammatory cell adhesion molecule P-selectin in thrombosis is critical. Recent data suggest that elevated levels of soluble P-selectin combined with a clinical examination favoring DVT has a positive predictive value for diagnosing DVT far exceeding that of d-dimer.11a,11b
Epidemiology
The incidence of recurrent, fatal, and nonfatal VTE has been estimated to exceed 900,000 cases annually in the United States.12 VTE has been estimated to occur with an incidence of approximately 1 per 1000 adult patients annually.13 This figure is supported by the 35-year population-based Rochester Epidemiology Project database of Olmstead County, Minnesota, which demonstrated an overall average age- and sex-adjusted annual incidence of VTE of 122 per 1,000,000 person-years.14 This landmark study also demonstrated higher age-adjusted rates in men compared with women (134 vs. 115 per 100,000 patients, respectively). First-time, or incident, VTE cases are estimated to occur in approximately 250,000 white individuals annually in the United States.15 However, there is a difference in ethnic groups diagnosed with VTE. African Americans demonstrate greater than a 25% increased incidence in first-time VTE compared with whites (103 vs. 141 per 100,000 patients). Conversely, the incidence of first-time VTE in both Hispanic and Asian or Pacific Islanders is significantly lower than in whites (62 vs. 104 per 100,000 patients; 29 vs. 104 per 100,000 patients, respectively).16,17 Nevertheless, the issue of VTE is not isolated to the United States. Recent estimates across the European Union have totaled nearly 700,000 cases of DVT, 435,000 cases of PE, and 543,000 fatalities.18
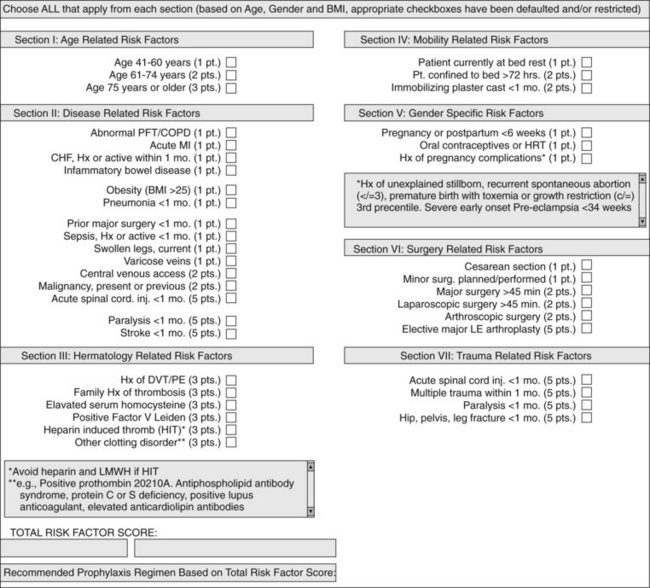
Although estimated incidences of VTE in both the community and hospital have been well documented, individuals at risk for VTE have been less well studied. Substantial differences have been noted in the distribution of risk factors between inpatients and outpatients.19 Extrapolating hospital inpatient data on the estimated 38 million patients discharged in 2003, 31% were considered to be at risk for VTE secondary to either major surgery or a medical illness.20 Although the incidence of VTE is multifactorial varying with the population studied, use of thromboprophylaxis, the intensity of screening, and the diagnostic test used, specific risk factors have been significantly associated with increasing VTE. Patients at highest risk are exemplified in the critically ill and trauma patients in whom bleeding risks prohibit thromboprophylaxis, and immobility contribute up to a greater than 80% DVT rate in specific populations.21 Risk stratification for VTE based on a number of individualized patient specific clinical attributes was initially proposed and validated by Caprini.22 In a large, single-center study, a modified Caprini risk assessment tool was applied to patients undergoing general surgery, associating higher scores with an increased incidence of VTE.23 Currently, electronic order entry to mandate a modified Caprini risk assessment tool should be performed for all hospitalized patients (Figure 47-1). Recognition of patients at high risk for VTE is a critical part in its prevention. In the next section, risk factors for VTE will be discussed in detail.
Risk Factors
Age
A higher incidence of VTE has consistently been associated with advanced age. In a community-based study of phlebographically documented DVT, the yearly incidence of DVT increased progressively from almost 0 in childhood to 7.65 cases per 1000 in men and 8.22 cases per 1000 in women older than 80 years, with the incidence of DVT increasing thirtyfold from 30 to 80 or more years of age.24 Similarly, Hansson and colleagues found that the objectively documented thromboembolic events in men increased from 0.5% at 50 years of age to 3.8% at 80 years.25
Immobilization
Immobilization promotes venous stasis and is a major risk factor for VTE. The prevalence of lower extremity DVT in autopsy studies parallels the duration of bed rest, with DVT occurring in 15% of patients dying after 0 to 7 days of bed rest as opposed to 79% to 94% of those dying after 2 to 12 weeks.26 Preoperative immobilization is associated with postoperative DVT, contributing a twofold higher risk.27 Patients with extremity paresis have a threefold higher risk for DVT and PE independent of hospital confinement.28
Travel
Despite the questionable importance of prolonged travel as a risk factor for VTE, in 2001 the World Health Organization acknowledged an association between air travel and VTE.29 Lapostolle and coworkers observed that over an 86-month period, 56 of 135.3 million airline passengers had severe PE. The frequency of PE in those who traveled more than 5000 km was 150-fold higher than those who traveled less than 5000 km.30 Paganin and associates observed a high incidence of VTE in patients with risk factors for DVT who traveled long distances. These investigators concluded that low mobility during flight was a modifiable risk factor for PE and that those with additional risk factors should increase their mobility.31
History of Venous Thromboembolism
Approximately 23% to 26% of patients with acute DVT have a previous history of DVT, and histologic studies confirm that acute thrombi are often associated with fibrous remnants of previous thrombi in the same or nearby veins.19,32,33 Depending on sex and age, population-based studies have demonstrated that recurrent thromboembolism develops in 1 of every 11 to 50 persons with a previous episode of thromboembolism, with the risk for recurrent thromboembolism being higher in patients with idiopathic DVT.34,35
Obesity
The evidence supporting obesity as a risk factor remains equivocal. In postmenopausal women, a body mass index of greater than 25 to 30 kg/m2 has been associated with significantly increased risk.36,37 Some investigators have reported obesity to be associated with a twofold greater risk for postoperative DVT,38 but multifactorial analysis by others has not shown obesity to constitute an independent risk.39 Obesity was not an independent risk factor for DVT in the Olmsted County study40 and has not been proved to be a risk factor for the development of DVT after stroke.41 Obesity has been found to be a risk factor, however, for recurrent DVT.40,42
Malignancy
Approximately 20% of all first-time VTE events are associated with malignancy.43 Either DVT or PE will develop in an estimated 1 in 200 individuals with malignancy, a fourfold higher risk than in those without malignancy.28 Considering all-cause in-hospital mortality for cancer patients, one in seven will die of PE.44 Correlation with location of the tumor reveals that the highest rates of VTE are associated with pancreatic malignancies, followed by kidney, ovary, lung, and stomach malignancies.45 Venous compression secondary to tumor growth, cancer-associated thrombocytosis, immobility, indwelling central lines, and chemotherapy or radiation therapy are all risk factors that increase the likelihood of developing VTE.46 Furthermore, as many as 90% of patients with cancer have abnormal coagulation parameters, including increased levels of coagulation factors, elevated fibrinogen or fibrin degradation products, thrombocytosis, and elevated levels of circulating prothrombotic microparticles.47–49
Surgery
All components of Virchow triad may be present in surgical patients: perioperative immobilization, transient changes in coagulation and fibrinolysis, and the potential for gross venous injury. In addition, surgery constitutes a spectrum of risk that is influenced by patient age, coexistent thrombotic risk factors, type of procedure, extent of surgical trauma, length of the procedure, and duration of postoperative immobilization.50,51 Approximately half of postoperative lower-extremity thrombi detected by 125I-labeled fibrinogen scanning develop in the operating room, and the remainder occur over the next 3 to 5 days.52 However, the risk for development of DVT does not end uniformly at hospital discharge. In one study, 51% of the thromboembolic events that occurred in patients undergoing gynecologic surgical procedures occurred after initial discharge.53 Similarly, up to 25% of abdominal surgery patients have been noted to have DVT within 6 weeks of discharge.54
Trauma
Despite improvements in trauma care and thromboembolism prophylaxis, DVT remains a significant source of morbidity and mortality in injured patients. In modern venographic series the incidence of DVT in the trauma patient is 58%.21 Recent trauma was the second most common risk factor for thromboembolism in the Olmsted County study by Heit and associates and was associated with a nearly thirteenfold increase in risk.55 As with postoperative DVT, several pathophysiologic elements may be responsible for the high incidence of DVT in trauma patients. Immobilization by skeletal fixation, paralysis, and critical illness are obviously associated with venous stasis, whereas mechanical injury is important after direct venous trauma and central venous cannulation. Less appreciated is the hypercoagulable state after depletion of coagulation inhibitors and components of the fibrinolytic system.56,57
Inherited Thrombophilia
Resistance to activated protein C was initially described by Dahlbach and associates in 1993.58 Subsequent studies from the Leiden University Hospital in the Netherlands revealed a mutation in the gene for factor V conferring resistant to cleavage by activated protein C.59,60 The factor V Leiden mutation has an autosomal dominant mode of inheritance and is at least tenfold more common than other inheritable defects.61,62 In a study of 4047 Americans, the carrier frequency of the mutation was 5.3% for whites, 2.2% for Hispanic Americans, 1.25% for Native Americans, 1.2% for African Americans, and 0.45% for Asian Americans, with no significant gender difference.63 The relative risk for first-time DVT is increased sevenfold in those who are heterozygous for the factor V Leiden mutation. However, the relative risk is increased eightyfold in individuals who are homozygous for the mutation.64
The prothrombin G20210A mutation, first described by Poort and associates in 1996, was demonstrated in 28 families with a documented history of VTE.65 Characterized by a transition from guanine to adenine at the 20210 nucleotide on the prothrombin gene, risk for VTE in the presence of the prothrombin G20210A mutation is threefold higher in heterozygotes than in wild type, and the presence of homozygosity further increases this risk.66 Although prothrombin G20210A is rare in individuals of Asian or African descent, additional investigations have shown that in those with spontaneous DVT, the incidence of the mutation may range from 7% to 16%, depending on the patient population studied.67,68
Protein C is a vitamin K–dependent serine protease that inhibits the coagulation system by inactivating factors Va and VIIIa.69 In a study of 10,000 healthy blood donors, the observed prevalence of inherited protein C deficiency was 1.45 per 1000.70 In a study of 2132 patients with VTE, 3.2% were found to have protein C deficiency.71 It is estimated that individuals who are heterozygous for protein C deficiency have a sevenfold higher risk for the development of VTE than the general population.72
Protein S is a vitamin K–dependent cofactor for the protein C–mediated inactivation of factors Va and VIIIa. Inheritance of protein S deficiency is autosomal dominant, and it is more common than protein C deficiency. The incidence of protein S–associated VTE has been reported to be 5% to 7%, with the prevalence in the general population being 0.13%.71,73,74 It is estimated that individuals who are heterozygous for protein S deficiency have an 8.5-fold higher risk for the development of VTE than the general population does.75
Antithrombin inhibits thrombin as well as factors Xa, IXa, XIa, and XIIa.76 Antithrombin deficiency is inherited in an autosomal dominant fashion. The majority of patients are heterozygous because homozygote patients usually die in utero. The incidence of VTE associated with antithrombin deficiency has been reported to be 0.5% to 3%, with a prevalence in the general population of 0.2%.71,76 However, it is estimated that individuals who are heterozygous for antithrombin deficiency have a twentyfold higher risk for the development of VTE than in the general population.77
Pregnancy
The incidence of VTE in the pregnant population is sixfold to tenfold greater than in nonpregnant controls.78 VTE accounts for approximately 10% of all maternal deaths.79 Studies that diagnosed DVT with venography, Doppler ultrasonography, or ventilation-perfusion scans for evaluation of clinically suspected thromboembolism have suggested an incidence of 0.029% to 0.055%.80 The risk for thrombosis appears to be twofold to threefold greater during the puerperium, with the highest incidence found after cesarean section.7,81–81b DVT in pregnancy has been attributed to impaired venous outflow secondary to uterine compression, and up to 97% of reported thromboses have been isolated to the left leg.81a Furthermore, pregnancy is associated with a transient hypercoagulable state because of increases in levels of fibrinogen; von Willebrand factor; and factors II, VII, VIII, and X.
Oral Contraceptives and Hormonal Therapy
Case-control and population-based studies have established the use of oral contraceptives as an independent risk factor for the development of DVT. Most studies have reported odds ratios of 3.8 to 11.0 for idiopathic thrombosis,82–85a with an unweighted summary relative risk of 2.9 in 18 controlled studies.83 Approximately one quarter of idiopathic thromboembolic events in women of childbearing age have been attributed to oral contraceptives.84 The risk of hospital admission for a thromboembolic event, including cerebral thrombosis, has been estimated to be 0.4 to 0.6 per 1000 for oral contraceptive users versus 0.05 to 0.06 for nonusers.82,86,87 Estrogenic compounds also increase the risk for VTE when used for suppression of lactation,88 in the treatment of carcinoma of the prostate, and as postmenopausal replacement therapy.89 Several studies have now reported a twofold to fourfold higher risk in women taking hormone replacement therapy.36,37,89–91 This increased risk is greatest during the first year of treatment.36,91
Central Venous Catheters
The use of central venous cannulation for hemodynamic monitoring, infusion catheters, and pacemakers has been associated with a rising frequency of DVT. This is particularly true for upper extremity thrombus, with as many as 65% being related to central venous cannulation.92 Although the incidence of symptomatic thrombosis may be low, studies using objective surveillance have reported thrombosis to occur at a mean incidence of 28% after subclavian cannulation.93 This risk also extends to femoral venous catheters; ipsilateral thrombosis develops in 12% of patients undergoing placement of large-bore catheters for trauma resuscitation.94
Inflammatory Bowel Disease
Clinical series have reported VTE to complicate inflammatory bowel disease in 1.2% to 7.1% of cases.95,96 Patients with Crohn disease have incidence rates of 31.4 and 10.3 per 10,000 person-years for DVT and PE, respectively. Ulcerative colitis was reported as having incident rates of 30.0 and 19.8 per 10,000 person-years for DVT and PE, respectively. Such thromboses frequently occur in young patients, are more common with active disease, and may affect unusual sites such as the cerebral veins.95,96
Systemic Lupus Erythematosus
In patients with systemic lupus erythematosus (SLE), lupus anticoagulant is present in 34%, and anticardiolipin antibodies are present in 44%, as compared with 2% for lupus anticoagulant and 0% to 7.5% for anticardiolipin antibodies in the general population.97 Among patients with SLE, those with lupus anticoagulant have a sixfold higher risk for VTE, whereas those with anticardiolipin antibodies have a twofold greater risk.98 The incidence of arterial or venous thrombosis is 25% in patients with lupus anticoagulant and 28% in patients with anticardiolipin antibodies.97 The relationship between antiphospholipid antibodies and thrombosis is much less clear in non-SLE disorders.
Anticoagulants, Including the New Agents
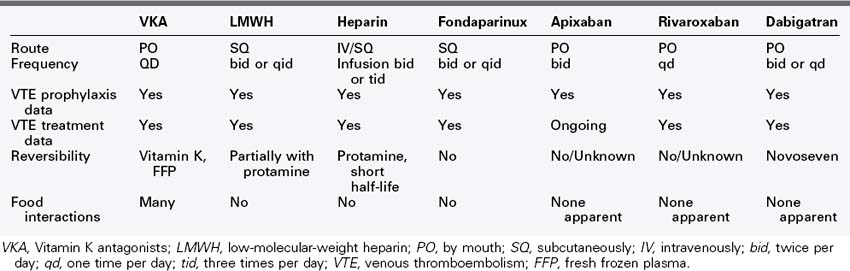
There are many pharmacologic options in current use for the treatment and prevention of venous thromboembolism (VTE). These medications include unfractionated heparin (UFH), low-molecular-weight heparin (LMWH), direct Xa inhibitors (anti Xa drugs), direct thrombin inhibitors (anti IIa drugs), and vitamin K antagonists (VKAs).99 UFH and directed thrombin inhibitors (such as argatroban and bivalirudin, which will not be discussed because they are not indicated for routine use) are used intravenously, requiring invasive administration and monitoring of dose effect. The LMWHs such as enoxaparin, and the pentasaccharide fondaparinux100 require subcutaneous administration but do not require monitoring. VKAs (e.g., warfarin) are the only oral anticoagulant approved by the U.S. Food and Drug Administration (FDA) for VTE. However, the use of VKAs requires frequent monitoring, and they have many interactions with medications and foods. Finally, some of these anticoagulants (e.g., UFH, LMWH) have known compounds capable of reversing their anticoagulant effects.101 In addition, new agents for treatment are undergoing testing, such as apixaban, rivaroxaban (Table 47-1), dabigatran, and edoxaban.
Low-Molecular-Weight Heparins
There are many LMWHs in clinical use; enoxaparin, dalteparin, and tinzaparin are approved for use in the United States.102 The class of LMWHs are defined most notably as being a more specific inhibitor to factor Xa than factor IIa, and the average molecular weight of the compound is 30% less than UFH.103 Clinical trial data has nearly unanimously revealed that LMWHs are as effective if not more effective than unfractionated heparin with their safety profile at least equivalent.103
A limitation to LMWH use is in those patients with renal dysfunction (creatinine clearance less than 30 mL/min). In these cases, effects are not predictable and safe dosing is difficult because LMWHs have the majority of their elimination by the kidneys.102 In addition, in an obese patient or another patient whose dosing appears complicated, anti-Xa activity can be measured to titrate therapeutic effect.102 The half-life of LMWH is longer, and as such may not be ideal for a high-risk patient with bleeding concerns, but in most other patient groups the LMWHs are viable and in many cases the current preferred method of anticoagulation for VTE.
Fondaparinux
Fondaparinux is a pentasaccharide that is in current use for the prevention and treatment of VTE with a subcutaneous delivery. Similar to LMWHs, fondaparinux is derived from UFH. It is based on the pentasaccharide moiety of the heparin molecule that selectively inhibits factor Xa via antithrombin, and it exhibits no endothelial or protein binding and is not thought to produce thrombocytopenia.102 Fondaparinux has been approved for VTE prophylaxis in total hip, total knee, and hip fracture patients, in extended prophylaxis of hip fracture patients, in abdominal surgery patients, and for the treatment of DVT and PE.104,105
Apixaban
Apixaban is an oral, direct factor Xa inhibitor by Bristol-Myers Squibb. The ADVANCE-1 study compared apixaban with a prophylactic dose twice daily of enoxaparin in total knee replacement patients. The bleeding rates were significantly lower in the apixaban group, but major bleeding rates were similar between compounds and they did not show noninferiority.106 The ADVANCE-2 trial compared apixaban with 40 mg daily of enoxaparin in the same population, concluding that apixaban was not inferior to the 40 mg daily of enoxaparin in VTE prophylaxis.107 ADVANCE-3 showed that apixaban compared with 40 mg daily of enoxaparin in hip replacement patients was not inferior in VTE prevention, while the bleeding events were not significantly different.108
Rivaroxaban
Rivaroxaban is an oral, direct factor Xa inhibitor sponsored by Bayer.109 RECORD-1 showed superiority of rivaroxaban and nonsignificantly different major bleeding events when comparing extended duration anticoagulation to 30 days for VTE prohpylaxis.109,110 The RECORD-2 trial examined extended duration rivaroxaban compared with standard short-term enoxaparin at 40 mg daily in patients undergoing hip replacement and again showed rivaroxaban to be superior.111 The RECORD-3 trial studied the same dosages, but both treatments were 14 days in duration in patients who had undergone knee replacements. This trial showed noninferiority of rivaroxaban with a similar safety profile.112 The RECORD-4 trial compared rivaroxaban (10 mg daily) to enoxaparin (30 mg twice per day) patients with knee replacements and again showed noninferiority with rivaroxaban with no statistical significant increase in bleeding.113
The EINSTIEN trial evaluated rivaroxaban versus standard anticoagulation in the treatment of acute DVT for standard duration.114 The primary outcome was recurrent VTE. The results indicated that rivaroxaban is statically not inferior to standard therapy in the treatment of acute DVT, without increased bleeding risk. The EINSTIEN group also added a continued treatment group to compare rivaroxaban with placebo for an additional 6 to 12 months after completion of their prescribed anticoagulation course. This study demonstrated a significant decrease in recurrent VTE, but there were four clinically significant bleeds in the treatment group and none in the placebo group, which did not reach statistical significance.114
Dabigatran
Dabigatran is an oral, direct thrombin inhibitor introduced by Boehringer Ingelheim (Ingelheim, Germany).115 Uniquely, dabigatran has some reversal with recombinant activated factor VII.115
The RE-MODEL trial was a VTE prophylaxis trial following total knee replacement. The trial showed that dabigatran was not inferior to daily enoxaparin at 40 mg once per day. 116 The RE-NOVATE trial in a hip replacement population also showed noninferiority.117 The RE-MOBILIZE trial showed dabigatran was inferior to 30 mg enoxaparin twice daily for postoperative VTE prophylaxis.118
The RE-COVER trial compared dabigatran to therapeutic anticoagulation VKAs (internal normalized ratio [INR] 2.0 to 3.0) in the treatment of acute VTE for 6 months. Dabigatran was not inferior in the 6-month rate of VTE recurrence and with similar bleeding rates. This important study showed that dabigatran can be effective in longer-term anticoagulation and had a similar safety profile to warfarin without the need for laboratory monitoring.119 Dabigatran is FDA approved for stroke and thromboembolism prevention in atrial fibrillation patents after similar effectiveness to warfarin was seen in the RE-LY trial.120
Future Anticoagulants
In addition to these anticoagulants, there are other promising compounds currently in preclinical development. One such example is soluble P-selectin/PSGL-1 inhibitors. In a series of nonhuman primate studies using a model of proximal venous thrombosis, inhibition of P-selectin or its ligand PSGL-1 was similar to LMWH in preventing and treating DVT.121 The P-selectin inhibitors reduce inflammation and therefore reduce thrombosis without inhibition of the coagulation cascade with less bleeding risks. Future clinical trials with these compounds are likely.
Length of Anticoagulation for VTE Treatment
Therapy for deep venous thrombosis is undertaken with the goals of reducing risk of pulmonary embolism, preventing extension of thrombus, and preventing thrombus recurrence. Immediate systemic anticoagulation should be achieved, as the risk of recurrent venous thromboembolism is significantly increased if anticoagulation is not therapeutic within the first 24 hours. In cases of suspected pulmonary embolism, this may necessitate heparinization before completion of diagnostic testing. Confirmation of DVT with duplex imaging is rapid and as a result usually precedes anticoagulation.122,123
Heparin
Historically, initial systemic anticoagulation has been undertaken with UFH with a loading dose of 80 U/kg and infusion rate adjusted to achieve activated partial thromboplastin time to 2 to 2.5 times normal. However, because of decreased rates of thrombotic events, decreased bleeding complications, decreased mortality (in cancer patients), and improved pharmacokinetic profile, LMWH is preferred over UFH for the initial treatment of acute DVT in most cases.123 LMWHs are dosed in a weight-based fashion and rarely require monitoring, except in circumstances such as renal failure, pregnancy, and morbid obesity. LMWH can be given subcutaneously once or twice daily with equal efficacy on an outpatient basis.
Vitamin K Antagonists
Oral administration of VKAs (warfarin being most common) is begun shortly after initiation of heparin therapy, because several days are usually required to bring the prothrombin time to an INR of 2.0 to 3.0. When initiating warfarin therapy, it is preferable to use a maintenance dose rather than a loading dose to avoid suppression of protein C. VKAs block the γ-carboxylation of several clotting factors, and prolongation of the prothrombin time beyond the range suggested is associated with a high incidence of bleeding complications. Nonhemorrhagic side effects are uncommon but include skin necrosis, dermatitis, and a syndrome of painful erythema in areas with large amounts of subcutaneous fat. Most changes are reversible if the drug is stopped, and the administration of fresh frozen plasma usually restores the prothrombin time. After an episode of acute DVT, anticoagulation should be maintained for a minimum of 3 months; some investigators favor 6 months for thrombi in the larger veins or after a second thromboembolic event. Many drugs alter the pharmacodynamics of warfarin by altering its metabolic clearance, rate of absorption, or inhibition of vitamin K–dependent coagulation factor synthesis or by altering other hemostatic factors. Phenylbutazone, sulfinpyrazone, disulfiram, metronidazole, and trimethoprim-sulfamethoxazole all potentiate the action of warfarin.124 Therefore, regular monitoring of prothrombin time is essential. In addition, levels of concurrent medications should be monitored, because warfarin may compete for binding sites, thus altering plasma levels of these drugs. Some foods that have high levels of vitamin K, such as broccoli, green leafy vegetables, and green teas, are also known to alter the effects of warfarin. Oral anticoagulants are teratogenic and should not be used during established or planned pregnancy. In a pregnant patient, LMWH is the drug of choice, and for long-term management, subcutaneous self-administration should be taught. This regimen allows a normal delivery and can be continued postpartum.
Duration of Anticoagulation
Anticoagulation following an episode of DVT should be continued until the benefits of preventing recurrent venous thromboembolism no longer outweigh the risks of anticoagulation.125 The major risks of recurrence depend on the patients risk factors for VTE and whether the initial episode has been appropriately treated.126 Major patient risk factors include reversible risk factors such as previous 1-month history of major surgery (general anesthesia longer than 30 minutes), or hospitalization longer than 3 days or cast immobilization of the lower extremity. In general, the greater the provoking risk factor, the lower the risk of DVT once anticoagulation therapy is stopped.127 Patients with modifiable risk factors should be treated for 3 to 6 months for a proximal (iliofemoral) or 6 to 12 weeks for a distal DVT. Shorter durations of therapy are associated with double the risk for recurrent DVT or PE within the next year.128–130
Patients with nonreversible risk factors include those with cancers and inherited molecular thrombophilias. Patients with cancer have an increased risk of recurrence and major bleeding complications compared with all other causes of DVT.131 Vomiting, malnutrition, and liver dysfunction, common among cancer patients, makes achieving consistent anticoagulation goals with oral VKA therapy challenging. Several randomized studies have been conducted comparing LMWH to oral VKA in this particular subset of patients; because of decreased mortality from bleeding complications and decreased risk of recurrence, initial treatment with LMWH is recommended with grade 1A evidence.132–134 These patients should be maintained on LMWH for the first 3 to 6 months and continued on anticoagulation until the cancer resolves.
Among patients with a thrombosis at an age younger than 40 years, a strong family history of VTE (two or more symptomatic relatives) or thrombosis at unusual locations, screening for hereditary thrombophilias is indicated.135 Screening of unselected individuals is not useful in predicting recurrence and should not guide therapy.127,136 Selected patients with clinically significant thrombosis and genetic defect may be maintained on anticoagulation indefinitely with periodic reassessments of the risk-to-benefit ratio of continuing oral VKA therapy. Heterozygote carriers of factor V Leiden and prothrombin 20210 gene mutations have only a modest risk of recurrence and should be treated according to standard recommendations.69 However, an individual who is a heterozygote carrier of both traits has an increased risk of VTE events and should be considered for lifetime anticoagulation.137
Patients with DVT in the absence of any identifiable risk factors are said to have an idiopathic DVT. Compared to patients with a reversible risk factor, recurrent VTE events are much more common in this patient population (≤3% vs. 10% at 1 year).138 Kearon and colleagues139 studied patients with unprovoked DVT, comparing 3 months of anticoagulation with indefinite anticoagulation. They demonstrated a 95% relative risk reduction in recurrence in patients receiving indefinite anticoagulation, but with a trend toward an increased risk of nonfatal major bleeding (p = 0.09). Because longer durations of anticoagulation reduced DVT recurrence but were associated with increased bleeding risk, Ridker and colleagues140 studied long-term subtherapeutic anticoagulation. The PREVENT investigators randomized patients after completing their full course of VKA anticoagulation (3 to 6 months) to either placebo or subtherapeutic VKA therapy (target INR of 1.5-1.9). At 2.1 years of follow-up, patients receiving indefinite subtherapeutic anticoagulation had a 62% relative risk reduction of recurrent DVT (14.6 vs. 5.5%; p < 0.001).
Three months of anticoagulation therapy is sufficient to decrease the risk of recurrent thrombosis related to the initial DVT. However, once therapy is discontinued, the risk for recurrence rises dramatically, with 30% to 50% of patients experiencing a recurrence at 10 years.141,142 Male gender, elevated d-dimer, incomplete resolution of DVT, body mass index of 30 kg/m2 or greater and postthrombotic syndrome are all associated with increased likelihood of recurrence.143 Additional anticoagulation therapy beyond 3 months is useful at preventing further episodes of recurrence not related to the initial event. Following the initial 3 to 6 months of therapy, all patients should be evaluated for long-term anticoagulation therapy.139 Similarly, patients with a recurrent VTE are at increased risk of additional episodes. In patients with a second DVT who were treated with 6 months of therapy, 20.7% experienced recurrent thrombosis, compared to 2.6% of patients treated indefinitely with anticoagulation.144 In high-risk patients, the risks of major bleeding during prolonged therapy (1.1% per patient-year), should be periodically weighed against the benefits of continuing anticoagulation. Advanced age (older than 75 years), history of gastrointestinal hemorrhage, noncardioembolic stroke, renal or hepatic disease, concomitant antiplatelet therapy, and poor control of anticoagulation increase the risk of bleeding complications.126
Two additional tests may be used to guide length of anticoagulation in selected patients. Follow-up duplex ultrasound screening identifies patients with residual thrombus. These patients have a twenty-fivefold greater risk of recurrence compared with patients who demonstrated recanalization of veins.142 A recent study demonstrated a 35% decrease in recurrent VTE events when duration of anticoagulation was guided by ultrasound evidence without any increase in bleeding complications.145 A second, better validated parameter is the d-dimer level, measured 1 month after completion of a standard oral VKA course. Patients with elevated d-dimer levels are prothrombotic and should continue to receive anticoagulation therapy.146
Fibrinolysis
Regional lysing with specialized catheters may be used to deliver recombinant tissue-type plasminogen activator directly to the thrombus. This technique can result in early thrombus resolution, with long-term valve preservation and a reduced incidence of postthrombotic syndrome.147–151 Mewissen and colleagues152 reported outcomes from a registry of patients with DVT treated with lytic therapy. Acute thrombosis completely lysed in 34% of cases, but at the expense of bleeding in 11% (but with a minimum of intracranial bleeding). One-year primary patency was 60%. Venous stents were used adjunctively in 104 of 473 patients. Other small studies have demonstrated the potential benefits of regional thrombolysis with improvements in vein patency153,154 and in the prevention of venous reflux.153
Surgical Therapy
Thrombectomy directly removes thrombi from the deep veins of the leg. Despite early efficacy, venographic follow-up often shows rethrombosis. However, when creation of an arteriovenous fistula is added to the procedure, patency is improved. Long-term follow-up is necessary to determine the incidence of postthrombotic syndrome, although small studies have demonstrated excellent efficacy.154,155 In the United States, this procedure is usually reserved for limb salvage in the presence of phlegmasia cerulea dolens and impending venous gangrene, although there are certainly cases in which surgical thrombectomy should be considered.
Vein Wall Abnormalities AFTER Deep Venous Thrombosis
Chronic venous disease (CVD) encompasses mild varicosities to disabling ulcers, which occasionally can progress to limb loss.156,157 The underlying pathophysiology is related to venous injury, valve damage, and obstruction; all of which culminate as venous hypertension when in the upright position.158 Venous hypertension leads to end organ damage, namely the skin and dermis. The sine qua non is hyperpigmentation, corona phlebectatica, lipodermatosclerosis, or frank ulceration of the limb.159 Once skin changes occur, dermal fibrosis and inflammation are present histologically.157 Factors involved include leukocyte activation with adhesion and emigration through the basement membrane with release of fibrotic growth factors and proteases. Dysregulation of iron may exacerbate this process.160
Postthrombotic syndrome (PTS) is a chronic sequelae of DVT, and typical symptoms include pain, heaviness, swelling, and cramping of the leg, which are generally worsened by standing and exercising.158 In advanced cases, venous ulceration occurs, causing additional pain and disability and increasing the cost of treatment.161,162 Postthrombotic morbidity has been reported to occur in 25% to 46% of patients following anticoagulation alone for acute DVT.8,158 The severity of PTS manifests relatively early after DVT and usually neither worsens nor improves, highlighted by a prospective multicenter cohort of 387 patients with acute DVT.9,163 Variables that predicted more severe postthrombotic morbidity included: severity of venous symptoms at 1 month; iliofemoral location; recurrent ipsilateral DVT; high body mass index; older age; and female gender.163 Thus, calf vein thrombosis is less often associated with PTS. Vein valve reflux plays a role in PTS, both in the affected deep segments and the superficial system.164
Definition of Postthrombotic Syndrome and Diagnosis
Unlike arterial diseases, PTS is more difficult to quantify. Expert consensus for PTS definition is the Villalta score, combining patient limb symptoms and signs in a graded scoring system165 (Box 47-1). The higher the score, the greater the severity of PTS (e.g., a score of greater than 15 suggests severe PTS). This system also allows for an evaluation of change in severity of disease over time.