Chapter 11
Venous Physiology
Lori L. Pounds, Lois A. Killewich
Anatomic Considerations
The understanding of venous anatomy has a rich history dating to Hippocrates (460-377 BC). In the field of phlebology, knowledge of anatomy is essential for evaluation and formation of a treatment plan. Venous anatomy is much more highly variable than arterial anatomy; moreover, the nomenclature is infused with duplicate and misleading names, which has led to the development of inappropriate treatment plans for patients. In 2001, an international interdisciplinary committee, under guidance of the presidents of the International Union of Phlebology and the International Federation of Anatomical Associations, formulated a consensus statement to update the Terminologia Anatomica (the official anatomic nomenclature).1 These changes, summarized in Table 11-1, represent an agreement to satisfy anatomists and clinicians, thus providing better care for the patient with a venous disorder.
Table 11-1
Changes in Nomenclature for the Superficial and Deep Veins of the Leg Based on the 2001 Conference
Venous Systems of the Extremities
The upper and lower extremity venous systems each contain three components: the superficial, deep, and perforating veins (Fig. 11-1).

Figure 11-1 Anatomy of the veins of the lower extremity. A, Superficial veins and perforators, anterior view. B, Superficial veins, posterior view. C, Deep veins, anterior view.
Lower Extremity Veins
Superficial Veins.
The superficial veins are located above the muscular fascia. In the legs, a subcomponent, the saphenous system, is present. The saphenous compartment2,3 is bounded superficially by a hyperechoic saphenous fascia and rests on the muscular fascia (the “Egyptian eye” seen on duplex imaging; Fig. 11-2). It contains the main great (formerly “greater”) saphenous trunk, accompanying small arteries, and the saphenous nerve below the knee. The saphenous fascia has been referred to in the past as the superficial fascia, the Colles or Scarpa fascia, or the subcutaneous pseudofascia. Since the consensus update, these terms are no longer recommended. The remaining superficial compartment below the dermis contains the accessory saphenous tributaries (anterior and posterior), which ascend parallel to the great saphenous vein. It also contains the communicating veins, a complex and variable system that connects with other veins in the same superficial compartment. An example would be the intersaphenous vein (formerly known as the vein of Giacomini), which connects the great and small (formerly “lesser”) saphenous veins. The reticular venous plexus and subpapillary venous plexus are also included in this area (Fig. 11-3).
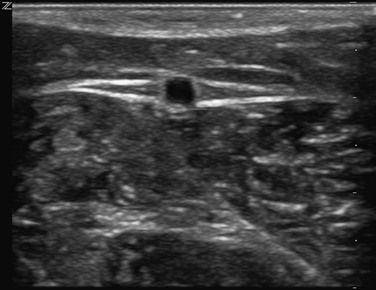
Figure 11-2 Transverse B-mode ultrasound image of the “Egyptian eye.” The pupil is the great saphenous vein; the eyelids are the superficial and deep layers of fascia surrounding the saphenous subcompartment.
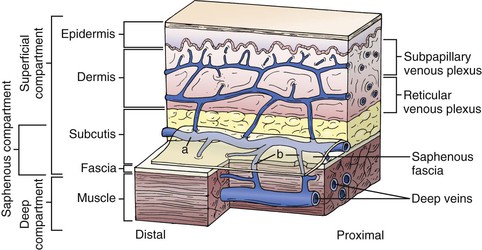
Figure 11-3 Relationship between the fascia and veins of the lower extremity. The fascia covers the muscle and separates the deep compartment from the superficial compartment. Superficial veins (a) drain the subpapillary and reticular venous plexuses and they are connected to deep veins through perforating veins (b). The saphenous fascia invests the saphenous vein. The saphenous compartment is a subcompartment of the superficial compartment. (From Mozes G, et al: New discoveries in anatomy and new terminology of leg veins: clinical implications. Vasc Endovasc Surg 38:367-374, 2004.)
Deep Veins.
The deep veins are located in the fascial muscle compartments, follow the same course as the arteries, and for the most part, have the same names. A significant change in the new terminology is that the superficial femoral vein is now named the femoral vein. Originally named because it was the venous equivalent of the superficial femoral artery, the name produced confusion for clinicians when reported that it was acutely thrombosed. Some would erroneously conclude that because it was the “superficial” femoral vein, the thrombus did not constitute deep venous thrombosis (DVT), and anticoagulation was not indicated. With the new terminology, reporting that the “femoral vein” is thrombosed should lead the practitioner to prompt initiation of anticoagulants, and possibly, thrombolytic therapy.
Perforating Veins.
There is a rich network of perforating veins that traverses the muscular fascia between the deep and superficial veins. These veins direct flow from the superficial to the deep systems for return to the heart via the calf muscle pump and a series of one-way valves. In general, the lower extremities have a series of large perforators located at 6-cm intervals from the base of the heel to the upper part of the thigh. Their names have been revised in the consensus document, and are also found in Table 11-1. The perforators may arise from the great saphenous or an accessory trunk (an indirect perforator) or connect a reticular vein directly to the deep system (a direct perforator). In the upper extremities, there are few perforating veins, which are found mostly in the arms connecting the basilic and brachial veins.
Upper Extremity Veins
Veins of the upper extremities do not function against gravity in the same fashion as the lower extremity veins do, and hence, do not contain as many valves. As in the lower extremities, deep veins accompany the arteries and have the same names. There are two main trunks of superficial veins: the cephalic and basilic veins. The cephalic vein courses on the radial (ventral) side of the forearm and on the lateral aspect of the biceps to the deltopectoral groove to join the axillary vein. The basilic vein is located on the ulnar (ventral) side of the forearm and traverses posterior to the elbow to join the brachial vein in the midportion of the arm. With the arms extended, the basilic vein is closer to the body, and the cephalic is closer to the head. There is a series of accessory ascending veins that parallel the main trunks and a series of communicating veins between the deep and superficial systems.
Venous Valves
Venous valves prevent retrograde flow of blood in a proximal-to-distal direction. They are found throughout the body, but the highest concentration is located in the lower extremities. Both superficial and deep veins contain valves. Anatomic studies have confirmed that they are present in all sizes of veins down to the level of venules.4 In general, the number of valves is higher in the more distal portions of the extremities and decreases proximally. Venous valves are most commonly bicuspid and can form sinusoidal dilatations, presumably from locally reversed flow.
Lower Extremity Venous Valves
The prevalence of valves in the iliofemoral venous system is low. Miles et al5 documented the location and extent in 70 necropsy specimens. They found no valves in the inferior vena cava and observed a mean of 1.2 valves in the iliofemoral system on the right and 0.97 valves on the left, with greater numbers in the common femoral and external iliac veins and fewer in the common iliac veins. Interestingly, no valves were found in the left common iliac vein in the area where it is crossed anteriorly by the right common iliac artery. The paucity of valves in the common iliac veins has been invoked as a possible reason why thrombolysis for iliofemoral venous thrombosis reduces the risk of developing postthrombotic syndrome. Obstruction, rather than valvular incompetence or destruction, is considered the primary cause for development of the postthrombotic syndrome following iliofemoral thrombosis.6
Moore et al7 reviewed the literature to document the number and location of venous valves in the femoro-popliteal segments in normal subjects. They found that valves were frequently present in the common femoral, femoral, and popliteal veins. A common femoral vein valve was observed in approximately 75% of cases, consistently located at the level of the inguinal ligament. The femoral vein contained one to four valves, and 90% of subjects had at least two valves. The most common location was just distal to the junction of the femoral and profunda femoris veins. The popliteal vein most commonly contained two valves, with one located just below the adductor hiatus and a second in the distal segment.
Upper Extremity Venous Valves
The location and number of valves in the subclavian and axillary veins was recently investigated by Celepci and Brenner8 in 59 limbs of 30 cadavers. These investigators found a terminal valve in the subclavian vein just distal to the venous angle (junction of the subclavian and internal jugular veins) in 100% of cases. In most cases, no additional valves were found in the subclavian vein. Three-quarters of axillary vein specimens possessed at least two valves, with the remainder possessing up to four valves. All valves in the axillary veins were found in the distal half.
Venous Wall Structure and Composition
Vein walls are composed of three layers—the intima, media, and adventitia. The intima is a single layer of cells resting on a thin layer of connective tissue. Valves are lined on both sides with a layer of intima over a thin connective tissue skeleton. Adjacent to the intima is the internal elastic lamina, which is more developed in larger veins and may be absent in small veins. The media consists of smooth muscle cells and connective tissue, such as collagen. In the great saphenous vein, it is thick and has a great capacity for muscular contraction. This conveys protection from dilatation and varicosity formation. In contrast, tributaries of the great saphenous vein have little media and are prone to the formation of varicosities. In the deep system, the content of collagen also varies greatly; calf veins have a large amount, which provides great wall strength and resistance to varicosity formation. The central veins have fewer smooth muscle cells and an increasing amount of connective tissue. The adventitia is not well differentiated from the media and contains loose connective tissue, vasa vasorum, and adrenergic nerve fibers.2
Normal Venous Hemodynamics
The venous system has two important functions—return of blood to the heart from the capillary bed and maintenance of cardiovascular hemostasis through changes in capacitance.9 In addition, these functions contribute to the peripheral pumping mechanism, which assists the heart in transporting blood during exercise and participates in the thermoregulatory system of the body.
Venous return is accomplished through the interactions of pressure gradients, muscle pumps, and valves. Pressure gradients dominate in the supine position, but in the upright position, gravity is counteracted by an efficient system of muscle pumps and valves.10–13 Unlike arteries, the volume of blood carried in a vein fluctuates considerably without concomitant changes in venous pressure. This large capacitance maintains cardiovascular stability.
Pressure-Flow Relationships and Venous Return
Throughout the human body, the pumping action of the heart causes movement of blood through both arteries and veins. The pressure generated by cardiac pumping is termed dynamic pressure. Under normal conditions in the supine position, blood flow is determined by dynamic pressure gradients, with arterial pressure being higher than venous pressure. The majority of dynamic pressure is dissipated in the arterial system before it reaches the capillary bed. At the venous end of the capillary bed, it ranges from 12 to 18 mm Hg. Atrial pressure averages 4 to 7 mm Hg under normal conditions. Hence, blood flows along this gradient and is returned to the heart.
In the upright position, venous flow in the lower extremities is dominated by the effects of hydrostatic pressure, which is derived from the weight of the column of blood below the right atrium (Fig. 11-4). A small effect of dynamic pressure is also present. Hydrostatic pressure is determined by the density of blood and the acceleration of gravity, and is expressed as a constant multiplier (0.77 mm Hg/cm) of the vertical distance in centimeters below the atrium.9 Return of blood from the dependent lower extremity to the heart requires overcoming the effects of hydrostatic pressure, which is accomplished by muscle pumps working in concert with venous valves. The leg muscle pumps, of which the calf pump is the most important, generate high pressure during muscle contraction, which propels venous blood toward the heart. During relaxation, valves close, and blood is prevented from refluxing down the leg and breaking up the hydrostatic pressure column.10,11 The negative pressure generated by valve closure also draws blood from the superficial to the deep systems via perforating veins, thus further enhancing return of blood to the heart.14 In the upright position under normal conditions, venous pressure measured on the dorsum of the foot ranges from approximately 90 to 120 mm Hg, depending on the individual’s height.

Figure 11-4 Effect of upright position on venous and arterial pressure. Pressure at the right atrial level is 0. In the supine position, hydrostatic pressure is essentially 0, so total intravascular pressure closely approximates dynamic pressure.
Upper extremity venous flow is governed by dynamic pressure gradients in a similar fashion to lower extremity flow. When the upper extremity is dependent (similar to the lower extremity flow in the upright position), pressure is governed not only by the distance below the atria but also by the distance between the atria and the first rib. This occurs because anatomy dictates that blood draining from the arm must travel vertically to the level of the first rib. When the arm is held above the atria in the upright posture, the upper extremity, head, and neck veins collapse.9
Venous Compliance and Capacitance
In addition to returning blood to the heart, veins play a significant role in maintaining cardiovascular hemostasis by storing large volumes of blood and by their ability to change shape and maintain pressure despite relatively large changes in volume.15 The venous system at times contains as much as 75% of the systemic blood volume. As such, reflex changes in vasomotor tone, together with passive distention or recoil of veins, provide mechanisms by which blood volume can be rapidly redistributed to compensate for loss of volume in conditions such as hemorrhage. For example, because of changes in venous capacitance, a healthy individual can tolerate an acute decrease in blood volume of up to 10% without serious cardiovascular consequences.16 A healthy individual can also compensate for large increases in total body fluid by using changes in venous volume and capacitance.
A complete discussion of the relationship between venous capacitance and cardiac output is beyond the scope of this chapter, and the reader is referred to several excellent reviews.17–19 A brief summary follows.
The relationship between pressure and volume at a given level of smooth muscle tone in the venous system is termed capacitance. Compliance is the change in blood volume that occurs for each unit of change in transmural pressure in a segment of vein; to put it another way, it is the slope of the capacitance curve.15,17–19
Venous capacitance is governed by the collapsible nature of the venous wall. A low volume of blood in a vein results in an elliptical shape with nearly coapted walls and low pressure (Fig. 11-5).9,19 A large increase in volume can occur with only a minimal increase in pressure. Eventually, the system is overwhelmed, and the vein dilates, which results in an increase in pressure per unit of volume. The difference between intraluminal pressure acting to expand a vein and tissue pressure acting to collapse the vein is termed transmural pressure. An increase in venous transmural pressure from 0 to 15 mm Hg results in an increase in venous volume of up to 250%. This corresponds to a change in shape from elliptical to circular (see Fig. 11-5).20 However, once the vein is circular, much higher pressure is required to stretch the venous wall to add additional volume. At arterial pressures, veins become as stiff as arteries.21–23
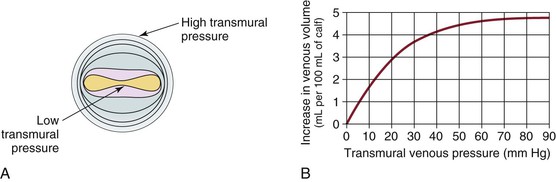
Figure 11-5 A, Cross-section of a venous lumen at various transmural pressures. At lower pressures the vein is elliptical, whereas at high pressures it is circular. B, Relationship of venous volume to transmural pressure. At low pressures, veins are compliant and change shape easily to accommodate large increases in volume. At high pressures, they become stiff and cannot accommodate large changes in volume. (A, Adapted from Moreno AH, et al: Mechanics of distention of dog veins and other thin-walled tubular structures. Circ Res 27:1069, 1970.)
Neural Regulation of Venous Capacitance and Compliance
Changes in venous capacitance are modulated both passively, by distention and recoil, and actively, by changes in the smooth muscle activity of vein walls. Smooth muscle activity is, in turn, controlled by the sympathetic nervous system.19–21 Passive transfer of blood out of an organ takes place when blood flow through the organ decreases. This occurs because the decrease in pressure across the resistance to venous flow is reduced, which follows from Ohm’s law applied to hydraulics (ΔP = F × R). An example is that splanchnic blood volume decreases to compensate for blood pooling in skin veins when body temperature rises during exercise.19
Active changes in capacitance occur in response to stimulation of the sympathetic nervous system. Studies performed in dogs by Brooksby and Donald showed that stimulation of the sympathetic system resulted in expulsion of approximately 70 mL of blood from the splanchnic circulation.24 Of this, the investigators determined that reduced flow across the splanchnic bed produced passive recoil of the venous system, which accounted for 45 mL of the blood expelled. The remaining 25 mL was expelled actively by stimulation of α-adrenergic receptors of the sympathetic nervous system.24
Arterial baroreceptors such as the carotid sinus also influence capacitance through modulation of the sympathetic nervous system. Decreased arterial blood pressure is sensed by the carotid sinus and results in secretion of catecholamines, which, in turn, increase smooth muscle activity in small veins and venules. Constriction of these vessels (reduced capacitance) causes venous blood to be transferred from the periphery to the heart, thereby boosting cardiac output.23
Effects of Respiration
Respiratory Effects on Lower Extremity Venous Return
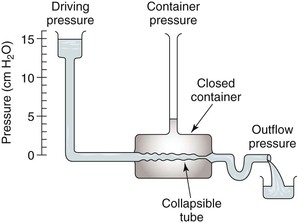
Respiration modulates venous return from the legs through alterations in venous capacitance and collapsibility related to movement of the diaphragm and changes in intrathoracic and intra-abdominal pressure. Sumner and Zierler25 developed a model of this relationship as shown in Figures 11-6 and 11-7. In this model, the pressure driving blood toward the heart (15 cm H2O) is represented by an elevated fluid reservoir. A collapsible tube, representing the inferior vena cava, passes through a closed container with a pressure of 5 cm H2O, representing the abdominal cavity. The end of the tube, which represents the right atrium, is open to the atmosphere, and therefore, has an outflow pressure of 0 cm H2O (see Fig. 11-6).
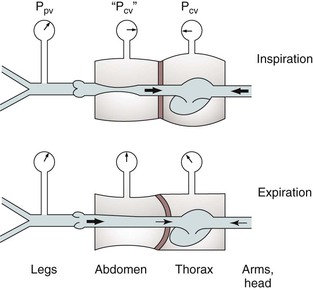
Figure 11-7 Effects of respiration on venous return. Pcv, Central venous pressure; Ppv, peripheral venous pressure. (From Sumner DS: In Bergan JJ, Yao JST, editors: Surgery of the veins, Orlando, FL, 1985, Grune & Stratton; pp 3-23.)
For blood to drain from the elevated fluid reservoir (the periphery) through the closed container (the abdominal cavity) to the heart, the pressure in the collapsible tube (the inferior vena cava) must rise until it exceeds that in the closed container, which allows the tube to open (increase in venous capacitance associated with a change in shape from elliptical to circular). This gradient is equal to 15 − 5 cm H2O or 10 cm H2O, rather than the gradient across the entire system, which is 1 to 15 cm H2O. Elevation of the driving pressure will increase the gradient, and hence, flow, but changes in outflow pressure have no effect unless the pressure rises above that in the closed container, in which case the gradient and flow will be decreased. Increasing pressure in the closed container will also decrease the gradient and flow. These relationships derive from the low pressure and “collapsibility” of veins, which means that flow cannot occur until the inferior vena cava, represented by the collapsible tube, achieves a transmural pressure greater than that in the abdominal cavity.
When inspiration occurs, the diaphragm descends, and intra-abdominal pressure is increased (see Fig. 11-7). This results in a decrease in the gradient, and venous return from the lower extremity is reduced. The increase is frequently large enough to reduce the gradient to 0, and venous flow ceases. This situation results in the well-described phenomenon of venous phasicity with respiration. Conditions that markedly increase venous pressure in the lower extremities augment the gradient to an extent that the effects of intra-abdominal pressure are overcome, and venous flow becomes continuous. This occurs in the setting of lower extremity venous thrombosis.
Respiratory Effects on Upper Extremity Venous Return
In the upper extremities (see Fig. 11-7), intrathoracic pressure, which is representative of outflow pressure in the model, is generally lower than extrathoracic, or driving, pressure. Hence, the gradient supports return of blood to the heart, and flow is less dependent on respiration. Conditions that alter this dynamic, such as congestive heart failure or pulmonary hypertension, cause central venous pressure to be elevated significantly above peripheral pressure, and the peripheral flow pattern is then derived from right heart function. This causes pulsatile flow patterns in veins of both the upper and lower extremities.
Calf Muscle Pump
Return of venous blood to the heart is facilitated by the action of muscle pumps in the legs, which occur in the thigh, in the calf, and in conjunction with the venous plexus on the plantar aspect of the foot.10,11,13,26 These pumps forcefully expel blood from the intramuscular and surrounding veins and propel it toward the heart (Fig. 11-8A). Ludbrook11 was the first to show the importance of these pumps and also noted that the calf pump appeared to be more important than the thigh pump. During vigorous exercise involving both the calf and thigh, 65% of the blood that entered the calf was expelled, whereas only 15% of the blood entering the thigh was expelled. At the same time, Stegall26 demonstrated that up to 30% of the energy required to circulate blood during vigorous exercise could be furnished by the muscle pumps. More recent evidence14 suggests that the calf muscle pump generates up to 200 mm Hg during muscular contraction and expels 40% to 60% of the venous volume of the calf (100 to 150 mL).
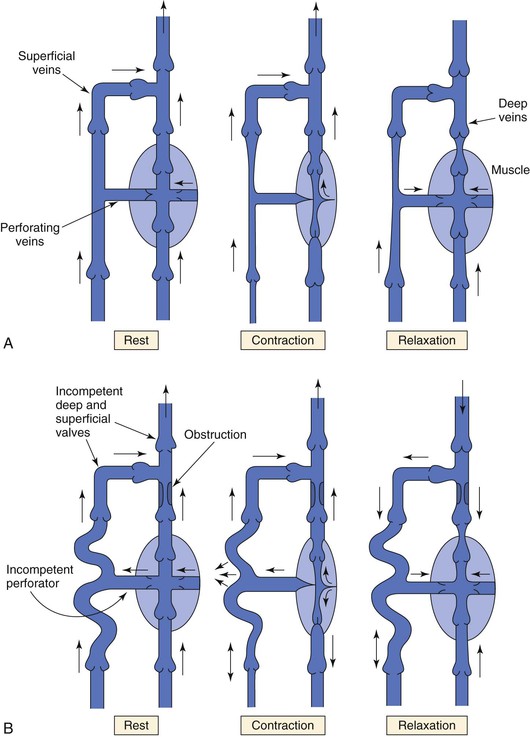
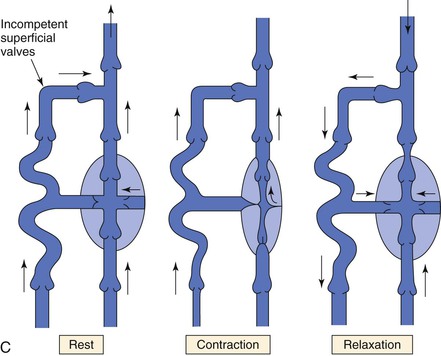
Figure 11-8 A, Dynamics of venous flow in response to calf muscle contraction in a normal limb. B, Dynamics of venous flow in response to calf muscle contraction in a postthrombotic limb with residual deep venous obstruction, incompetent valves, and secondary varicose veins. Blood flow in perforating veins is bidirectional. C, Dynamics of venous flow in response to calf muscle contraction in a limb with primary varicose veins. (From Sumner DS: Venous dynamics—varicosities. Clin Obstet Gynecol 24:743-760, 1981.)
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree