Central venous obstruction encompasses the varied disorders that result in the compression and occlusion of the venous structures in the chest. These include diseases related to the superior vena cava (SVC) as well as those affecting its contributing branches.
CENTRAL VENOUS ANATOMY
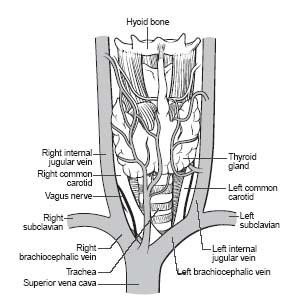
The SVC is formed from the union of the right and left brachiocephalic/innominate veins (Fig. 26.1). Each brachiocephalic vein is formed from the union of the respective internal jugular and subclavian veins. The subclavian veins represent the continuation of the brachial and axillary veins from each upper extremity. The anatomic structure delineating the transition from axillary to subclavian vein is the first rib.
ETIOLOGY OF CENTRAL VENOUS OBSTRUCTION
Central venous obstruction is typically divided into two broad categories based on the anatomic location of the obstruction—that is, the SVC or the axillary and subclavian veins. Brachiocephalic obstructions are inconsistently included in either of these groups.
SVC obstruction, which frequently involves the brachiocephalic branches, results in the SVC syndrome. Malignant causes, particularly carcinoma of the bronchus, are now responsible for in excess of 90% of cases of SVC syndrome (1–5). Squamous cell carcinoma of the bronchus is more commonly associated with SVC syndrome than non-squamous cell-type cancers. Less common malignancies associated with SVC syndrome include lymphoma, metastatic disease, and thymoma.
Axillary/subclavian venous occlusion may be due to primary or secondary causes. The dominant primary cause is Paget–von Schroetter syndrome (PSS) (also referred to as effort thrombosis thoracic outlet syndrome [TOS]) (6). This syndrome most commonly occurs in the dominant arm (~70%) of males (~70%) and is related to repetitive or unusual arm activity. There is a high incidence of associated anatomic abnormalities that lead to compression of the thoracic outlet (7,8) (Fig. 26.2). In 1821, Sir Astley Cooper first described axillary/ subclavian artery symptoms due to compression from a cervical rib (9). In 1875, James Paget described the clinical symptoms resulting from subclavian vein thrombosis (arm swelling and pain). In 1884, von Schroetter correctly attributed these upper extremity venous symptoms to thrombosis or compression of the subclavian vein at the thoracic outlet (7–9). Consequently, venous thrombosis at the thoracic outlet became known as venous TOS or PSS.
Axillary/subclavian venous occlusion is most commonly caused by secondary etiologies such as indwelling central venous catheters or pacemaker leads, underlying malignancy, hypercoagulability, trauma, or infection. Several investigators have shown that venous obstruction after pacemaker implantation may be observed in approximately 31% to 50%, though it may be closer to 20% when pre- and post-lead studies are reviewed (10). These secondary causes also frequently result in central venous obstruction of the SVC.
DIAGNOSIS
Axillary/subclavian venous occlusion is supported by the presence of upper extremity swelling, venous engorgement, and pain. Secondary causes such as pacemaker leads or central venous catheters will usually be clinically obvious. These signs and symptoms, in association with radiologic documentation of venous compression at the thoracic outlet, confirm the diagnosis of TOS/PSS. Sometimes, venous compression cannot be demonstrated, and the diagnosis is made clinically by the pattern of venous thrombosis.
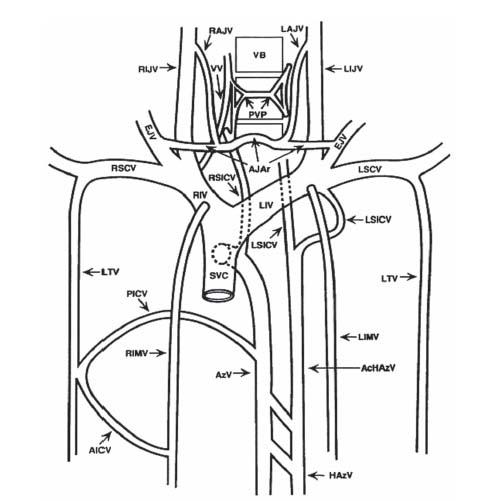
Signs and symptoms of SVC occlusion include difficulty breathing, swollen neck and chest wall veins, bilateral arm/ neck/facial swelling, hoarseness, and headache. CTA and other noninvasive imaging modalities (including multiplanar fast MR imaging) are very helpful in diagnosing central venous obstruction (Fig. 26.3). Digital subtraction angiography (DSA) remains the gold standard and typically reveals the site of occlusion and the development of collateral pathways. An obstructed SVC initiates collateral venous return to the heart from the upper half of the body through four principal pathways (Fig. 26.4). The first and most important pathway is the azygous venous system, which includes the azygous vein, the hemiazygous vein, and the connecting intercostal veins. The second pathway is the internal mammary venous system plus tributaries and secondary communications to the superior and inferior epigastric veins. The long thoracic venous system, with its connections to the femoral veins, and the vertebral veins provide the third and fourth collateral routes, respectively. Despite these collateral networks, venous pressure is almost always elevated in the upper chest in the presence of SVC obstruction (Fig. 26.5A–C).
Figure 26.1 • Schematic illustration of central venous anatomy.
MANAGEMENT
Recanalizing Central Venous Obstruction
In managing patients with central venous obstruction, heparinization is often the first step in relieving symptoms from the thrombosis; although it does not lyse the clot, it will help increase collateral flow to reduce the edema experienced by many patients. Second, if there is an offending mechanical factor contributing to thrombosis/obstruction (i.e., central line and pacemaker lead), it should be removed if possible. Unfortunately, many lines and wires cannot be removed.
Before interventional options are considered, recanalization must first be achieved. Once femoral and/or jugular venous access is achieved with ultrasound guidance, the authors typically place a large sheath and deliver either diagnostic catheters, such as a 5-Fr. vertebral or multipurpose, or a 6- or 7-Fr. guide catheter to the site of the occlusion. We frequently use a 0.035″ glidewire to cross the occlusion, starting with the floppy glidewire and moving to the stiff shaft glidewire if necessary. Sometimes, recanalization is the most difficult part of the procedure as the clot may be chronic and organized. Failure to cross central venous occlusions can account for 15% of the technical failures (11). Flush occlusion of the central vein increases the risk of failure.
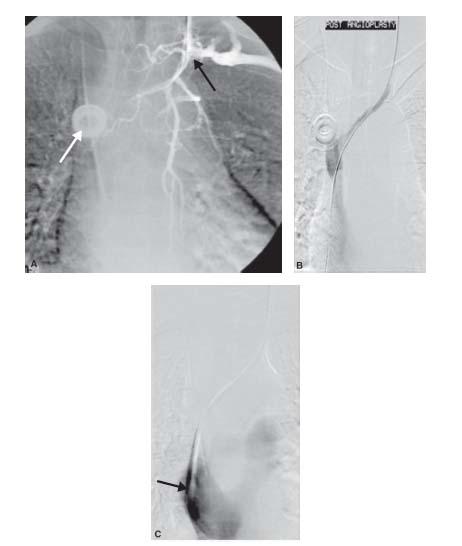
If the vessel is partially occluded, then thrombolysis prior to an attempted intervention may be an option. Thrombolysis has a rate of complete lysis of 76% to 84% for primary axillary/subclavian occlusions (12–14). Unfortunately, urokinase (formerly Abbott Pharmaceuticals, Abbott Park, IL) was more effective in treating older thrombus than the currently available thrombolytic agent, tissue plasminogen activator (tPA) (Figs. 26.6 to 26.8).
Managing Axillary/Subclavian Venous Disease
Patients with primary (i.e., PSS) and secondary thrombotic occlusion of the axillary/subclavian veins have distinct management strategies. In patients with PSS, the thrombotic occlusion is typically crossed and thrombolytic therapy is administered in the hopes of achieving recanalization and some restoration of venous flow. For occlusions older than 10 to 14 days, the chances of successful thrombolysis are poor. Some studies have found residual lesions in as many as 100% of these patients following thrombolysis (15,16). Regardless of the outcome following thrombolysis, prompt surgical correction of the underlying anatomic abnormality is required. Typically, the surgery will involve resection of the first rib, division of the costoclavicular ligament, and division and resection of the anterior scalene muscle. This surgical approach has proved to be an effective treatment (15). The timing of surgical decompression following thrombolysis is controversial: most surgeons wait at least 4 weeks before performing a decompressive procedure.
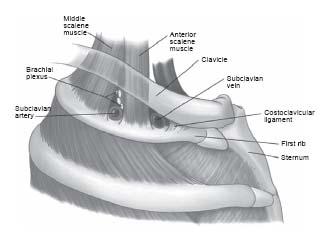
Figure 26.2 • Schematic illustration of the normal anatomy of the thoracic outlet. Note the relationship of the subclavian vein to the anterior scalene muscle posteriorly, the costoclavicular ligament anteriorly, the clavicle superiorly, and the first rib inferiorly. (From Urschel HC Jr, Patel AN. Surgery Remains the Most Effective Treatment for Paget-Schroetter Syndrome: 50 Years’ Experience. Ann Thorac Surg. 2008;86:254–260).
Figure 26.3 • Schematic illustration of major collateral networks that compensate for the presence of central venous obstruction. Ac-HAzV, accessory hemiazygos vein; AICV, anterior intercostal vein; AJAr, anterior jugular arch; AzV, azygos vein; EJV, external jugular vein; HAzV, hemiazygos vein; LAJV, left anterior jugular vein; LIJV, left internal jugular vein; LIMV, left internal mammary vein; LIV, left innominate vein; LSICV, left superior intercostal vein; LSCV, left subclavian vein; LTV, lateral thoracic vein; PICV, posterior intercostal vein; PVP, paravertebral plexus; RAJV, right anterior jugular vein; RIJV, right internal jugular vein; RIMV, right internal mammary vein; RIV, right innominate vein; RSCV, right subclavian vein; RSICV, right superior intercostal vein; SVC, superior vena cava; VB, vertebral body; VV, vertebral vein. (Reprinted from Chasen MH, Charnsangavej C. Venous chest anatomy: clinical implications. Eur J Radiol. 1998;27:2–14, with permission from Elsevier.)
In patients with secondary occlusion of the brachiocephalic, subclavian, and/or axillary venous segment(s), the most common treatment options include angioplasty and/or stent placement: angioplasty with 0.035″-compatible balloon catheters with diameters ranging from 5 to 15 mm (Figs. 26.5 and 26.6). Non-compliant balloon catheters are preferred. Most operators size the balloon to the vessel being dilated. Cutting balloon catheters should be considered for resistant lesions. In general, endovascular therapy with PTA or cutting balloon catheters for central venous stenosis is safe, with low rates of technical failure. However, patency rates with balloon angioplasty are poor with reported 12-month primary and secondary patency rates of 22% to 29% and 63% to 73%, respectively (17,18). As a result, multiple additional interventions are the rule with both treatments. Cutting balloon angioplasty has not been shown to improve patency rates compared with PTA and does not add to the longevity of ipsilateral hemodialysis access sites (18).
If angioplasty fails, we will consider stent placement. For lesions in the brachiocephalic, axillary, or subclavian veins, self-expandable stents (e.g., Wallstent) are recommended (Fig. 26.9). General rules for stent choice and placement include the following: avoid undersizing the stent, avoid placing brachiocephalic or subclavian stents across the internal jugular vein junction, and avoid placing brachiocephalic stents across the junction with the SVC. By blocking these key junctions makes future central venous catheter placement, particularly of large diameter hemodialysis catheters, very difficult (19). Patency rates following stenting of subclavian/axillary veins is highly variable, with 6- to 12-month patency rates of 29% to 88% being reported in the literature (19–21).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree