INDICATIONS AND PIVOTAL TRIALS
Natural History of Renal Artery Stenosis
The prevalence of renal artery stenosis (RAS) in patients with hypertension (HTN) has been estimated at 1% to 5%; however, angiographic studies suggest this may be an underestimate (1). Among patients with documented atherosclerotic disease in other peripheral vascular beds, the prevalence of RAS may be as high as 30% to 40% (2).
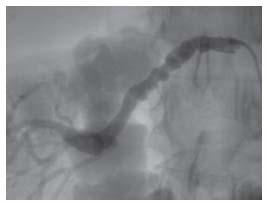
Fibromuscular dysplasia (FMD) and atherosclerosis are the two primary pathologic etiologies of RAS. FMD accounts for about 10% of all RAS cases, typically occurs in younger (i.e., less than 50 years) women, and has the classic “beads on a string” appearance at angiography (Fig. 22.1). A young woman with new-onset HTN that is accelerating or refractory to antihypertensive therapies suggests the diagnosis of FMD.
Atherosclerosis is responsible for approximately 90% of all RAS cases and generally occurs in an older population (i.e., greater than 50 years) with vascular risk factors. There is no gender preference observed in atherosclerotic RAS, and these patients may present in a myriad of ways: refractory or accelerating HTN, worsening renal function, angiotensin-converting enzyme (ACE) inhibitor-induced renal failure, flash pulmonary edema, or as an incidental finding during other testing.
The natural history of RAS depends largely upon the underlying etiology. FMD may involve the intimal, medial, or adventitial layer of the vessel, but medial FMD accounts for the overwhelming majority of cases (i.e., about 90%) (3). Progressive narrowing occurs in one third of patients with medial FMD; however, progression to complete occlusion is an extremely rare event. In contrast, atherosclerotic RAS is a progressive disease that may culminate in occlusion of the vessel. In one series, patients with RAS of less than 60% severity had a rate of progression to significant disease (i.e., greater than 60% severity) of approximately 20% per year. Among patients with vessels who had an initial stenosis of greater than 60% severity, there was progression to complete occlusion in 5% of patients at 1 year and 11% at 2 years; however, some investigators have been unable to demonstrate a relationship between stenosis severity and renal function (4–6). While one might conclude from this that stenoses do not cause renal dysfunction, such a conclusion would be unfounded. Fundamentally, the kidney requires blood flow to function. Thus, it is absolutely clear that severe stenoses and occlusions yield a nonfunctioning kidney.
Why then is there difficulty relating stenosis severity to function? Clearly, there are factors beyond the degree of stenosis that influence function. Some are intrinsic to RAS, including the duration of the insult, atheroemboli, hypertensive nephrosclerosis of the contralateral kidney, activation of the renin–angiotensin system, and finally the characteristics and effects of the stenosis (including lesion length, minimal lumen diameter, etc.) on renal blood flow and intrarenal pressure. Additionally, other factors, such as essential HTN, diabetes, concomitant medications, generalized atherosclerosis progression, and aging, play roles in determining overall renal function.
Renal Artery Revascularization
An early, nonrandomized comparison suggested a benefit of surgical revascularization of severely narrowed renal arteries; however, surgery has been associated with significant perioperative complications and mortality (7). Most patients with RAS have lesions in other vascular beds, making them high-risk operative candidates; therefore, percutaneous transluminal renal angioplasty (PTRA) became an attractive alternative. Notably, the results of PTRA and surgery appear equivalent, when compared directly (8). PTRA was associated with improved blood pressure (BP) control and a decrease in need for antihypertensive medications in retrospective studies; however, PTRA is associated with a high restenosis rate for atherosclerotic lesions involving the ostium, which is the location of 80% to 85% of all atherosclerotic RAS lesions. With the advent of stents, the problems associated with angioplasty may be circumvented.
Rees et al. reported 96% technical success rate with Palmaz® stents in ostial lesions (9), and perhaps more importantly, stents appear to be superior to PTRA, when compared directly (10). For these reasons, stents have become the dominant mode of revascularization for ostial-atherosclerotic lesions of the renal artery.
DATA SUPPORTING PERCUTANEOUS RENAL ARTERY REVASCULARIZATION
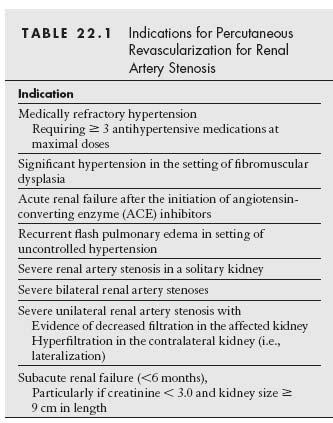
The primary goals of treatment in RAS are to improve the management of refractory HTN and to preserve or improve renal function (Table 22.1).
While there is general agreement that PTRA/stent procedures offer benefits for patients with the aforementioned conditions, who are found to have bilateral renal artery stenoses, the indications for PTRA/stent procedures in similar patients with unilateral disease are more contentious.
In three randomized trials that compared balloon angioplasty to medical therapy for the treatment of HTN in patients with RAS, there was no difference in BP control or serum creatinine among the two treatment groups; however, the need for fewer antihypertensive medications in patients treated with angioplasty was a consistent finding in each of the studies (11–13). These trials were limited by the high rate of crossover in the treatment arms (i.e., from medical arm to angioplasty arm)—the small number of patients enrolled, the treatment of patients with stenoses that may not have been physiologically significant, and the use of angioplasty without stenting. The recently published Angioplasty and Stenting for Renal Artery Lesions (ASTRAL) trial represents the first randomized controlled trial (RCT) that compared contemporary renal artery revascularization using stents plus optimal medical therapy versus optimal medical therapy alone (14). In this trial, 806 patients diagnosed with RAS in which the physician was unclear of the clinical benefit of renal artery revascularization were enrolled. The primary end point of the study was the change in renal function over time, measured as the slope of the reciprocal of serum creatinine. There was no significant difference in the two treatment groups. Likewise, there were no differences noted in the two treatment groups with regard to the prespecified secondary end points that included BP control, time to first renal event, time to first major cardiovascular event, and mortality. This trial has been heavily criticized by proponents of renal artery revascularization (15). The major criticisms include the decision to enroll patients with an uncertain clinical indication for renal revascularization, which clearly makes it difficult to demonstrate a clinical benefit. The choice of the rate of decline in renal function as the primary end point is also problematic, given that 25% of patients in the study had normal renal function and an additional 15% had near-normal renal function. A significant proportion of patients in the study did not appear to have a physiologically significant stenosis, with 40% of patients being documented to have ≤70% stenosis of the renal artery. With the absence of any core lab for the study, it would appear likely that this represents an underestimate. Finally, the quality of the interventional operators in this study is questionable, given the high rate of major adverse events (9%) when compared with other contemporary renal artery stent studies (~2%). For this reason, it seems fair to conclude that the major finding of the ASTRAL trial is that routine renal artery revascularization is not indicated in patients without a clear clinical indication. The ongoing Cardiovascular Outcomes in Renal Atherosclerotic Lesions (CORAL) trial does address some of the shortcomings of the ASTRAL trial and will be pivotal in determining the future role of renal artery revascularization.
Figure 22.1 • Right renal artery angiogram in a 40-year-old female demonstrating “beaded” appearance of vessel lumen, consistent with a diagnosis of fibromuscular dysplasia.
PATIENT SELECTION
In the absence of clear data from RCTs guiding treatment decisions in patients with RAS, the interventionist must determine the appropriateness of renal artery revascularization for each individual patient who presents for evaluation. In essence, one must ask two fundamental questions: (1) Is the patient’s clinical condition being caused by RAS? and (2) having fulfilled that condition, does the patient have a reasonable chance of clinical benefit from revascularization? It is important to emphasize that a patient’s clinical condition may be caused by RAS, but he or she may not benefit from revascu-larization. For example, a patient may have a critical stenosis of a renal artery that results in profound ischemic changes in the affected kidney resulting in atrophy of the kidney. If the indication for revascularization is restoration of renal function, then revascularization of an atrophic kidney is unlikely to provide the patient with any clinical benefit and the patient should be managed medically.
In attempting to answer these two critical questions, the synthesis of data from a number of sources is required including clinical history and exam, serum Cr (including the trajectory of the serum Cr over time), urinary protein, and imaging data. The presence of a clear clinical indication is important. If a young woman is diagnosed with RAS due to FMD, then the clinical indication driving the need for intervention will be the presence of HTN. Likewise, in patients with RAS due to atherosclerotic disease, the following clinical indications should be present before considering renal artery revascularization: refractory HTN, ACE inhibitor–induced renal failure, recurrent flash pulmonary edema in the setting of HTN, unexplained hypokalemia, subacute (i.e., 6 months) onset of renal insufficiency, or an incidental finding of decreased kidney size on imaging studies obtained for other reasons (16).
The serum Cr is an important variable that should be examined in patients with atherosclerotic RAS. As mentioned previously, the patients who typically stand to benefit from renal artery revascularization in terms of improvements or stabilization of renal function include those with a recent deterioration in renal function. The more acute this deterioration (assuming the absence of other etiologies), the more likely the patient will recover renal function following revascularization. A patient who presents with significant fluctuation in renal function over a long period of time is unlikely to benefit, as the underlying etiology of the fluctuation in renal function is unlikely to be due to RAS. The limitations of serum Cr as a marker of renal function should be accepted, however, particularly in patients with unilateral RAS. In such cases, as disease progresses in one kidney, there is often lateralization or hyperfiltration of the unaffected kidney, which maintains the serum creatinine within a relatively normal range despite significant renal impairment in the affected kidney.
Urinary protein measurements are a crude method of assessing the parenchymal function of the kidney(s). Urinary protein measurements > 1 g/24 hr suggest significant parenchymal disease and make it less likely that revascularization of the main renal artery will result in a significant clinical benefit.
Imaging data may be derived from purely anatomic studies (i.e., CT and MR angiography) or from ultrasound that provides both anatomic and important functional data. Anatomic assessments of the severity of RAS are important, as most physiologically significant stenoses will be in the severe to critical range, and as a result are more likely to have a clinical response to revascularization. The vertical kidney size provided by these modalities is also an important variable as it serves as a crude assessment of the health of the kidney parenchyma. Most experts would agree that a kidney size 8 cm suggests severe parenchymal disease with minimal effective residual renal function and should raise the threshold for revascularization.
Much has been written regarding the value of the resistive index (RI) measurements from the renal parenchyma in determining the clinical response to renal revascularization. The RI is calculated as 1 – EDV/PSV (EDV, end-diastolic velocity, and PSV, peak systolic velocity) from Doppler waveforms sampled from the superior, mid, and inferior segments of both kidneys. A high RI (i.e.,>0.8) has been regarded as an indication of severe parenchymal disease and as a marker of a lack of clinical response to revascularization. However, this measurement needs to be examined critically, as it can be influenced by a variety of factors. For example, the presence of a severe main RAS will result in a parvus tardus waveform (i.e., reduction in the PSV measurement), thus resulting in a reduction in the RI measurement. Thus, a patient with concomitant severe parenchymal disease and severe main RAS could have a normal RI measurement based on the balance of these two influences on the RI. Additional variables that can influence the RI measurement include the presence of valvular heart disease (e.g., aortic stenosis—decrease, aortic regurgitation—increase, and stiff calcified aorta—increase). Unfortunately, the RI is poorly understood by many interventionalists and general medicine physicians. It should be viewed in the context of the entire body of information gathered from the patient and its limitations should be clearly understood.
A typical screening and treatment algorithm for RAS is outlined in Figure 22.2.
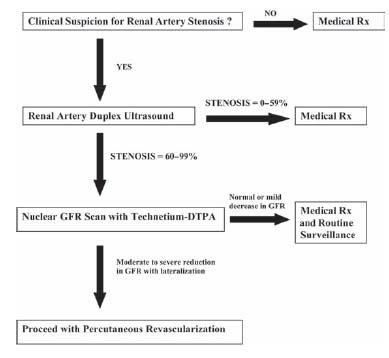
Figure 22.2 • Algorithm for diagnosis and management of patients with suspected renal artery stenosis.
Paired renal arteries arise from the lateral aspect of the abdominal aorta at the level of the first and second lumbar vertebrate, just inferior to the anterior origin of the superior mesenteric artery. The origin of the right renal artery is often slightly higher than that of the left renal artery. The main renal artery remains intact for a variable length, prior to subdividing into segmental arteries. These segmental arteries further subdivide into interlobar arteries that subsequently divide into the arcuate and interlobular arteries. The arcuate and interlobular arteries provide the smaller arterioles that penetrate the renal cortex and medulla (Fig. 22.3).
There are a number of variations in renal artery anatomy that warrant mention. The most common variant is the presence of an accessory renal artery, which occurs in up to 30% of individuals and refers to a second, generally smaller-caliber, renal artery that most commonly arises inferior to the main renal artery (Fig. 22.4). Accessory renal arteries typically arise from the abdominal aorta but may also arise from the common iliac, superior and inferior mesenteric, and right hepatic arteries. In some instances, the accessory renal artery may be of similar caliber to the main renal artery, thus supplying a large portion of the renal blood supply. In these circumstances, revascularization of stenosed accessory renal arteries has been performed.
A second anatomic variant is early subdivision of the main renal artery (Fig. 22.5). Normally, a main renal artery remains intact for several centimeters prior to dividing into a variable number of segmental branches. In some cases, a main renal artery may immediately subdivide into segmental branches just beyond its origin, thus making optimal percutaneous revascularization more challenging.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree