Indications
Number
Upper extremity venous duplex to rule out thrombosis
191
Upper extremity dialysis evaluations
142
Upper extremity radial artery bypass harvest
16
Upper extremity graft surveillance
35
Evaluation for line placement
9
Upper extremity arterial duplex
8
Upper extremity symptoms with/without cold challenge
35
Thoracic outlet evaluation
6
Total
442
A total of 701 upper extremity venous duplex scans to rule out thrombosis were analyzed. Some 38% were positive for thrombosis and isolated superficial venous thrombosis was identified in 85 patients. Distribution and sites of veins are presented in Table 39.2. When considering the anatomic distribution of acute UEDVT, the subclavian and internal jugular veins were the most commonly involved sites. Multiple patients have been reported to have concomitant deep and superficial system involvement [15]. The rate of asymptomatic catheter-related DVT is high and may be lowered by correct initial positioning of the central venous catheter tip either positioned in the superior vena cava or at the junction between the right atrium and the superior vena cava [16].
Table 39.2
Veins involved in thrombosisa
Veins involved | Total |
|---|---|
Subclavian | 103 |
Cephalic | 99 |
Internal jugular | 78 |
Axillary | 59 |
Brachial | 56 |
Basilic | 61 |
External jugular | 19 |
Radial | 6 |
Antecubital | 8 |
Ulnar | 3 |
Total | 492 |
Patients with suspected UEDVT should undergo duplex scanning. Diagnostic accuracy of upper extremity duplex scanning is very good with sensitivity and specificity of 78–100% and 82–100% [10]. Accuracy can be improved by having the patient perform Valsalva maneuvers, the sniff test, or by utilizing further tests. Duplex ultrasound may also detect axillary and cervical lymphadenopathy possibly secondary to malignancy. Compression is limited with poor visualization of the proximal subclavian vein. The brachiocephalic vein and the superior vena cava are beyond the clavicle and sternum [10]. CT scanning may detect a central thrombus as well as extrinsic compression of the vessel. However, it has the disadvantage of requiring a contrast load and has not been fully validated. Magnetic resonance studies accurately detect central thrombus and provide a detailed evaluation of collaterals and blood flow; however, it has limited availability, the patient may be claustrophobic and it may be not be suitable in some patients with implants (see Table 39.3) [17].
Table 39.3
Advantages and disadvantages of imaging modalities used to diagnose upper extremity deep vein thrombosis
Advantages | Disadvantages | |
|---|---|---|
Ultrasound | Inexpensive | May fail to detect central thrombus that is directly below the clavicle |
Noninvasive | ||
Reproducible | ||
CT scan | May detect central thrombus | Contrast dye |
May detect the presence of extrinsic vessel compression | Not fully validated | |
Magnetic resonance | Accurately detects central thrombus | Limited availability |
Provides detailed evaluation of collaterals and blood flow | Claustrophobia | |
Not suitable for some patients with implanted metal |
Anatomy
Veins of the arm drain via the brachial or cephalic system. The brachial vein flows into the axillary vein, which is then joined by the cephalic vein to form the subclavian vein. The axillary vein is contiguous to the brachial vein traveling in the tunnel formed by the clavicle and subclavius muscle, anterior on the scalene muscle laterally, the first rib posterior-inferiorly, and the costo-clavicular ligament medially. In the subscapular region, the axillary vein receives both the subscapular and suprascapular veins. There are many anastomotic collateral pathways that can potentially bypass the axillary subclavian venous system. They may be divided into the shoulder to chest wall, the shoulder to ipsilateral anterior neck, the shoulder to ipsilateral posterior neck, and the shoulder to contralateral neck. In the event of axillary vein thrombosis, collaterals will usually develop from the shoulder to the chest wall. If the thrombosis extends to the subclavian vein, the collaterals generally develop to the posterior ipsilateral neck or from the shoulder to the contralateral neck [18] (see Fig. 39.1).
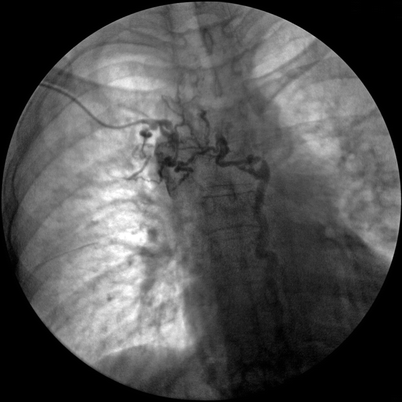

Fig. 39.1
Chronic subclavian occlusion with well-developed collaterals
Thoracic outlet syndrome (TOS) refers to compression of the neurovascular bundle including the brachial plexus, subclavian artery, and subclavian vein as it exits the thoracic inlet delimited by the scalene muscles, the clavicle, and the first rib. Cervical ribs, musculofascial bands, and clavicular first rib anomalies have also been described as other causes. Symptoms include a combination of vascular and neurologic findings [19].
Duplex Imaging Technique
The upper extremity examination begins with a detailed assessment of signs, symptoms, past medical history, and risk factor analysis. The patient is then placed in a supine position and the radial vein is visualized from the wrist to the brachial vein. Next, the ulnar vein is followed from the wrist to the antecubital fossa; where the ulnar and radial veins form the brachial vein; the brachial vein is followed into the upper arm. At the junction of the basilic and brachial veins, the axillary vein is formed. The axillary vein is followed under the shoulder in the direction of the clavicle. The junction of the cephalic and axillary veins forms the subclavian vein. The junction of the subclavian and jugular veins originating at the innominate vein is not visualized routinely because of its depth; however, Doppler signals analyzed in this area provide indirect information about the patency of the central veins and newer color scanners have increased the ability to image them. Doppler signals of the subclavian vein are assessed. Normally, the flow is spontaneous and phasic with augmentation, and no reflux is identified. The internal jugular vein is examined from the clavicle cephalad until it dives under the mandible. Doppler signals of the internal jugular vein also are obtained to assess spontaneous and phasic flow. Finally, the superficial veins are assessed by following the basilic vein along the ulna until it joins the brachial vein. The largest-diameter antecubital vein connects the basilic and cephalic systems at the antecubital fossa. The antecubital perforator also is seen connecting the cephalic and brachial veins. The cephalic vein is traced along the radius and up the arm, where it joins with the axillary vein. The antecubital, cephalic, and basilic veins are the superficial veins of the upper extremity.
For reporting convenience and ease of communication, the upper extremity is divided into zones (see Fig. 39.2). In each examination, the position of the probe and localizing abnormal findings are reported. Each zone covers approximately 10 cm in length. Zone 1 is located at the suprasternal notch, Zone 3 at the acromial clavicular process, Zone 5 at the anticubital fossa, and Zone 8 at the wrist. Zones are used to report thrombus location allowing accurate comparison between studies and technologists.


Fig. 39.2
Zones of reference for upper extremity venous scanning: midline = 1.0, acromion = 3.0, elbow = 5.0, wrist = 8.0, and fingertips = 9.0 (Adapted from Lohr [14]. With permission from Elsevier)
When a visible intraluminal thrombus is identified, several of its characteristics are assessed to determine its relative age. These characteristics include clot occlusiveness, clot retraction, clot distention, vein compressions, echogenicity and homogeneity, the development of collateral venous channels, and recanalization. The clot may be partially or totally occluding. A totally occluding clot indicates an acute process. Free-floating thrombi actually are tethered distally but extend cephalad in the vein without a more proximal attachment to the vessel wall. These free-floating thrombus tails exhibit a side-to-side waving in the venous lumen that can be induced by gently bouncing the probe on the skin or with respiration. Free-floating tails usually become attached to the venous wall within 1–2 weeks. Characteristics of thrombi help age the thrombus (see Table 39.4).
Table 39.4
Clot characteristics: relative value in clot aging
Characteristic | Acute value | Chronic value | ||
|---|---|---|---|---|
Degree of occlusion | Total | *** | Partial | *** |
Free-floating | Free | **** | Stationary | ** |
Clot retraction | Retracted | *** | Adherent | *** |
Clot distention | Distended | *** | Contracted | ** |
Clot compressibility | Soft | **** | Firm | * |
Surface character | Smooth | ** | Irregular | * |
Echogenicity | Faint | * | Bright | * |
Homogeneity | Homogeneous | ** | Heterogeneous | ** |
Collaterals | Absent | * | Present | **** |
Recanalization | Absent | * | Present | **** |
Because of the limited ability to compress the deep venous system, especially in the area where the subclavian passes beneath the clavicle, technical modifications such as the use of adjunctive procedures and color flow duplex analysis are critical for correct assessment. In the areas where the vein may not be accessible to compression color Doppler, it is important to pay close attention to color flow gain settings to avoid oversaturation, which may obscure small intraluminal clots or areas of incomplete thrombosis [20].
It is important to document the full extent of the disease including the contralateral neck and proximal arm. Radionuclide venography may be of further benefit to diagnose Port-A-Cath thrombosis in clinical practice [21]. Duplex ultrasonography is the method of choice for the initial diagnosis of patients suspected with thrombosis of the upper extremities. However, in patients with isolated flow abnormalities, contrast venography should be performed [22]. Imaging pitfalls to avoid include the cephalic vein being diagnosed as the axillary vein. Probable occlusion of the internal jugular vein may also occur and mirror image artifact may result in the appearance of two subclavian veins in the supraclavicular region [23].
The use of duplex imaging of the upper extremity has increased dramatically. In the instance of dialysis access, upper extremity evaluations increasingly are being requested before placement. Preoperative evaluation of dysfunctional dialysis grafts allows operative planning [24]. Vein mapping improves graft durability in patients with disadvantaged outflow and vein size, and continuity can be established. The protocol implemented in the author’s vascular laboratory evaluates both veins and arteries (see Fig. 39.3). Blood flow in the superficial palmar arch is assessed at baseline with radial and ulnar comparison. In addition, calcifications and aberrant arterial anatomy are identified. Veins are evaluated using tourniquet distention if less than 3 mm at rest.

Fig. 39.3
Evaluation of the arteries and veins protocol
Venous Abnormalities on Duplex Exam
Clot retraction is defined as the concentric separation of the thrombus from the vein walls; there appears to be a very thin gap between the thrombus and the circumference of the venous wall. Retraction is thought to occur within a few hours of thrombus formation through clot contraction of the platelet fibrin mesh formation and extrusion of serum. Clot retraction usually lasts only 1–2 weeks, and then the clot becomes adherent to the vein wall.
Clot distention occurs when the vein is dilated to a larger-than-normal diameter by a thrombus in a cross-sectional area. In this context, clot distention differs from venous distention caused by obstruction or by venous hypertension in the absence of an intraluminal thrombus. In the latter, it is possible to completely collapse the wall of the vein with the pressure of the probe and receive a Doppler signal. Veins exhibiting clot distention gradually shrink over several weeks to months.
An acutely thrombosed vein may be partially compressible. Unlike a chronically thrombosed vein, acute thrombi can be deformed by only light probe pressure. When viewed transversely, the round vein appears oblong. A thrombus remains soft for approximately 24 h after formation. The clot surface may be smooth or irregular; this usually is best assessed by a longitudinal view of the thrombus tip. Acute thrombi tend to have smooth, rounded tips because of continued surrounding flow (Fig. 39.4a, b).
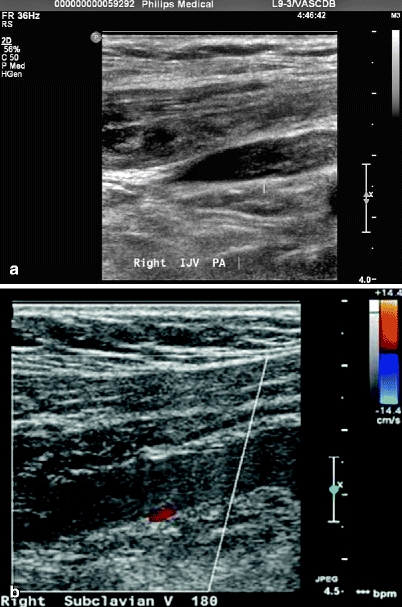

Fig. 39.4
(a) Acute internal jugular vein partially occlusive DVT. (b) Subclavian vein occlusion
Echogenicity is defined as the overall brightness of the clot compared with the surrounding tissues. Brightly echogenic thrombi tend to be chronic because serum reabsorption makes them denser. Apparent changes in echogenicity may be influenced by electrical gains of the instruments, depth of the structure being assessed, and acoustic shadow of the overlying tissues, making echogenicity a somewhat subjective finding. Homogeneity is also assessed. Acute thrombi tend to be homogeneous, whereas chronic thrombi tend to be heterogeneous.
Development of collateral venous channels is an absolute sign of chronic thrombosis. These channels are very small, lie parallel to the main vein trunk, and do not contain valves. Normal venous tributaries are larger, enter the main venous channel at an acute angle, and contain valves. Collateral venous channels are best visualized in a transverse field and may be seen as early as 1–2 weeks after initial thrombosis; however, they usually are not visible for a month or more after venous occlusion.
Recanalization is evidenced by an open, collapsible channel that runs through a thrombus. The recanalized channel, which is surrounded entirely by a clot, tends to occur rather late.
Using the aforementioned characteristics, thrombi are classified as acute, chronic, or indeterminate. Classification must be based on the characteristics of the entire thrombus rather than on an isolated segment because the deep venous thrombosis is a continuing process. For this reason, a single thrombus may manifest various aging characteristics in different regions. Repeat duplex scans are often necessary and commonly show the evolution of characteristics that are equivocal with the initial scans.
Upper Extremity DVT in Children
Venous thrombolic events are recognized as a growing clinical problem in children. The incidence of these events in prospective population based studies reported by International Registries (Canadian, German, and Dutch) revealed the presence of at least one associated underlying medical condition in most cases. Among all of the registries, the presence of a central venous line was the most common etiologic factor. In children more than 50% of the upper extremity venous thromboembolic events are due to the presence of central venous lines. Spontaneous venous thromboemboli are rare in children and usually involve the renal veins in the neonatal period or the lower extremity venous system in the older age groups [25–28].
Soft-Tissue Abnormalities on Duplex Examination
Some scans are performed to evaluate soft-tissue abnormalities, which can be differentiated by their characteristics. The features of a pseudoaneurysm include visible intraluminal swirling and turbulent Doppler spectra. Further, common associated findings include a centrifugal thrombus with a superimposed arterial spectrum and a visible arterial laceration or communication.
Abscesses, on the other hand, have hyperechoic contents, give off no Doppler signal, have inducible eddy motions and often have an echo-dense capsule. Commonly associated findings include regional solidification.
Hematomas exhibit a variable echo density, are non-compressible, have no fluid movements, or clear margins and give off no Doppler signal. If hematomas are less than 12 h old, they may be hypoechoic with focal liquefaction. If they are more than 2 weeks old, dissection through the soft tissues is commonly seen.
Edema fluid is radiolucent, follows tissue planes, and is not compressible. Lymph nodes are spherical and encapsulated with a mixed internal echogenicity, and are noncompressible. Small lymph nodes may be invisible or difficult to see. Large lymph nodes are seen in the presence of infection, cellulitis, or tumors. In fact, the duplex scan image of large, swollen lymph nodes shows architecture strikingly similar to the cross-section of a lymph node that is depicted in pathology textbooks.
Cysts are slightly echogenic and are best seen in a transverse view, where they can be distinguished from the surrounding tissue structures. Cysts may be partially compressible and at times, may compress an artery or vein, causing an increase in peak velocity signals.
Duplex scanning holds special value for the evaluation of aneurysms, pseudoaneurysms and for the surveillance of grafts. In the past 5 years, there has been rapid expansion in the use of duplex imaging for the diagnosis and evaluation of upper extremity venous thrombosis, arterial suitability, and soft-tissue abnormalities and for the evaluation of grafts. Requests for examination of the upper extremity have increased and this area of clinical interest likely will continue to expand (Fig. 39.5).
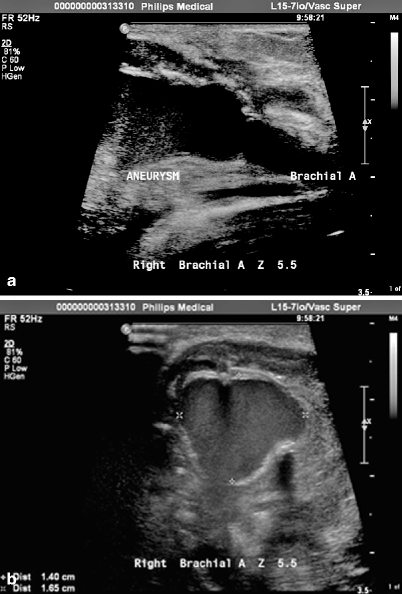

Fig. 39.5
(a) Transverse and longitudinal view of brachial artery aneurysm, zone 5.5. (b) Aneurysmal segment measures 1.40 × 1.65 cm. Note the normal diameter vessel to the right of the image
A multicenter collaborative study that assessed the accuracy of lower extremity duplex scanning reported that the procedure was 97% sensitive in extremities with positive phlebograms [29]. However, the phlebogram was negative in 191 extremities and was considered to be incorrect in 6 instances. Thus the phlebogram was not a perfect standard when negative, and the duplex scan was slightly more accurate [30, 31].
It has not been possible to generate similar data for the upper extremity because there is a blind area behind the clavicle. Thus a negative duplex scan does not absolutely rule out thrombosis of the subclavian vein. In the author’s study, a phlebogram was requested when there was doubt; unfortunately, only 10 were obtained. Although all were confirmatory, it is possible that some subclavian vein thromboses were missed. In most cases, however, a negative duplex scan and normal Doppler signals in the subclavian vein were accepted as evidence of normal venous flow. Rapid-sequence spiral CT scanning offers another modality to evaluate this area.
In the author’s vascular laboratory, all scans are performed with commercially available high-resolution duplex instrumentation. Both black-and-white and color scanners are used. The interpreting physician grades all scans for quality using a three-part system:
Poor indicates that a portion of the scan was not interpretable or a segment of the extremity was not visualized.
Fair indicates that diagnosis could be made but the entire extremity was not well visualized.
Good indicates that all structures were well visualized.
Because duplex scanning has become the standard of practice to evaluate extremity veins, grading and assessment are an important means to ensuring the accuracy and reliability of a vascular laboratory.
To perform at the level required for ICAVL certification, a vascular laboratory must conduct phlebography in less than 10% of patients. Because duplex scanning is significantly dependent on the technologist’s skill, quality control issues are central to continuing validation of this test. Formal comparison with phlebography is no longer available in this laboratory. When possible, blinded duplicate scanning programs by different technologists are used as a method to document quality control and improve test reliability. Further, physicians who are responsible for interpreting findings and overseeing the vascular laboratory must be personally skilled in the techniques of venous duplex imaging and the use of equipment. Finally, better scanner resolution also has improved the results of venous duplex imaging [32–37].
Catheter-induced thrombosis is an increasingly common event because the central veins are used more often for access, nutrition, chemotherapy, and monitoring. In the author’s study, the most common associated risk factor for upper extremity thrombosis was a central or peripheral venous catheter. Indwelling catheters and malignancy were the most commonly combined risk factors; however, no identifiable risk factors were present in 10% (11/107) of patients with thrombosis. Hyperalimentation (TPN) was infused in 27% of patients with thrombosis. Further, screening and venography demonstrate that 30–60% of patients with central venous catheters will have a thrombus in the axillary subclavian venous segment, and 3% of these patients will develop clinically symptomatic subclavian vein thrombosis. Pulmonary emboli have been reported in 5–12% of a series of upper extremity thrombosis and it is estimated that 1% of these patients will die. Major vein thrombosis in the upper extremities, much like that in the lower extremities, may spread from a superficial vein into the deep system; therefore, upper extremity surveillance of isolated superficial vein thrombosis (SVT) is recommended [38, 39].
Comparison of Upper and Lower Extremity DVT
Thrombosis of the upper extremity has distinct characteristics when compared to the venous thromboembolism of the lower extremity. The American DVT Registry Database enrolled over 5,000 consecutive adult patients with acute DVT between October 2001 and October 2010, which reported approximately 11% of patients with UEDVT. The database included catheter-related venous thromboembolic disease of the upper extremity (6% of total cases), noncatheter-related venous UEDVT (5% of total cases), and lower extremity DVT (89% of total cases) [40].
When compared with lower extremity DVT, UEDVT patients were younger, less often white, leaner, had a lower body mass index, and most frequently presented with edema and less commonly with extremity discomfort, dyspnea, or chest pain [41]. Patients with UEDVT also had symptomatic PE less frequently (3% vs. 16%, P < 0.001) [40]. The differences reported in comparison of lower extremity DVT in the upper extremity includes patients less frequently had a family history of venous thromboembolism (18% vs. 31%, P = 0.06) or concomitant PE (8% vs. 31%, P = 0.001). Additionally, they were less frequently carriers of Factor V Leiden (12% vs. 30%, P = 0.009) and had lower thrombin generation marker levels [41].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


