Fig. 54.1
(a) 3D gray scale (colorized) image of carotid artery atherosclerotic disease in the distal CCA with a small surface ulceration. (b) Corresponding B-mode (2D) image showing small plaque ulceration initially seen in 3D reconstruction
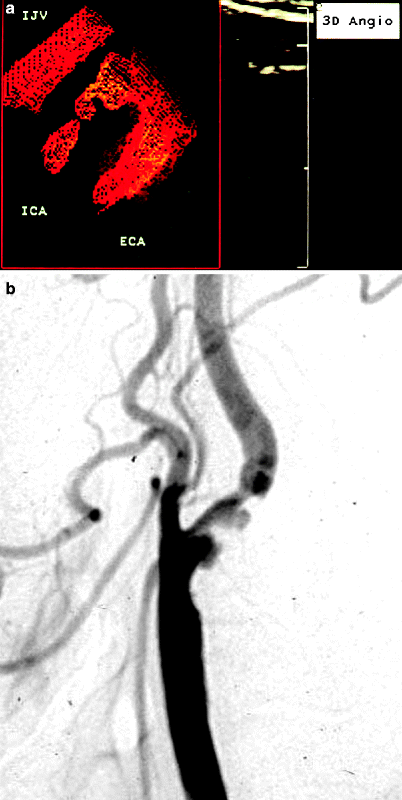
Fig. 54.2
(a) 3D power Doppler reconstruction of a carotid artery bifurcation showing an ulceration in the lesion just past the origin of the internal carotid artery (ICA). IJV internal jugular vein, ECA external carotid artery. (b) Corresponding arteriogram showing ulcerated plaque in proximal ICA
3D Power Doppler Angiography
Bendick et al. [23] evaluated the accuracy of 3D power Doppler angiography in the carotid artery bifurcation compared with digital subtraction contrast angiography and surgical findings at carotid endarterectomy. Thirty-two patients were studied, with 64 vessels available for correlation. Luminal narrowing was categorized according to standard gradations of percent stenosis or total occlusion, using direct measurements from the 3D images without knowledge of the angiographic results and applying the methodology of stenosis measurement of the NASCET study (Fig. 54.3). In addition, surface morphology of the 3D images was evaluated to determine the extent of luminal narrowing, defined as focal, moderate (<1 cm), or lengthy (>1 cm), as well as the presence of plaque ulceration. Three carotid bifurcations had atherosclerotic lesions that were too heavily calcified for adequate power Doppler angiography and could not be classified by 3D imaging. Of the remaining 61 bifurcations, 53 were accurately classified as to percent stenosis, giving an overall accuracy of 87%, similar to results obtained when Doppler velocity criteria are used to categorize lesion severity. The sensitivity of 3D imaging to a >50% diameter stenosis was 100%; the positive predictive value for >50% diameter stenosis was 81% (21 of 26 bifurcations) (Fig. 54.4). All four total occlusions were correctly identified by 3D imaging.
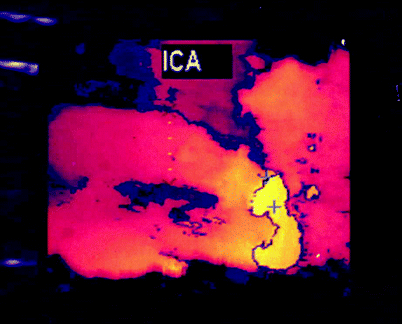
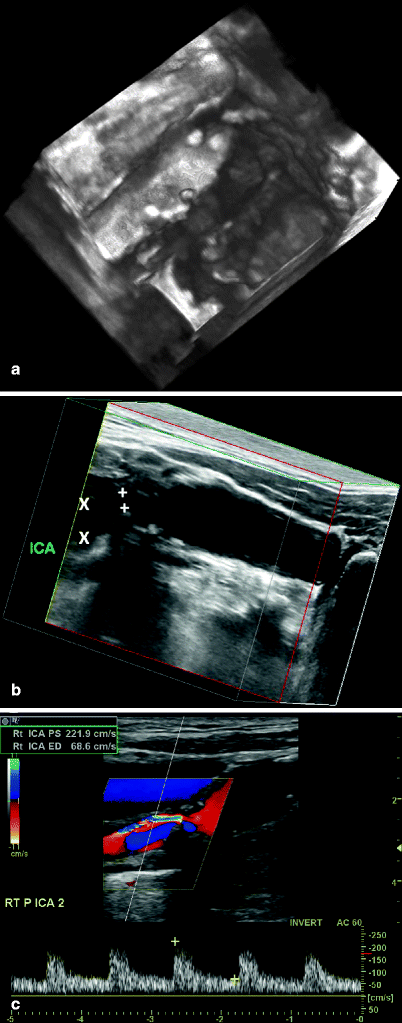

Fig. 54.3
3D reconstruction of a carotid bifurcation region applying the NASCET methodology for stenosis measurement, with ultrasonic calipers on the internal carotid artery showing the minimal lumen diameter (X) and the distal ICA lumen diameter (+). Also note the small ulcerative crater at midlesion

Fig. 54.4
(a) 3D reconstruction of a carotid bifurcation region showing the coronal view (not obtainable with 2D imaging) of a complex, irregular atherosclerotic lesion at the origin of the ICA. (b) 3D reconstruction of a carotid bifurcation region showing a 50–70% diameter reduction in the proximal internal carotid artery (ICA); the minimum lumen diameter was 2.5 mm (+) and the distal ICA lumen measured 5.8 mm (×), a 58% diameter reduction. (c) Spectral Doppler data for the lesion shown in (b) with a peak systolic velocity of 221 cm/s and an end-diastolic velocity of 68 cm/s, corresponding to a 50–70% diameter reduction
In the evaluation of the extent of lesions, 11 of 13 (85%) were correctly classified as focal, 24 of 29 (83%) as being of moderate extent of <1 cm, and 14 of 14 (100%) as extended lesions of >1 cm. Five lesions were considered to be ulcerated by 3D angiography (Fig. 54.2) with four of these ulcerations shown by subtraction angiography. 3D power Doppler angiography was believed to provide an accurate noninvasive technique comparable to subtraction angiography for the anatomic evaluation of carotid bifurcation atherosclerotic disease, with selectable viewing projections that helped eliminate vessel overlap and other artifacts. The technique complemented the hemodynamic data already available from conventional 2D duplex ultrasound, but at this time could not replace it; instead, it allowed a more thorough evaluation of any obstructive disease present without significant additional testing.
Delcker et al. [24] investigated 3D power Doppler angiography for transcranial imaging of the major intracranial vessels. Previous studies have shown improved ability for transcranial duplex ultrasound to detect and image the intracranial vessels using power Doppler compared with color Doppler imaging [25, 26]. Delcker et al. also studied the use of a transpulmonary-stable ultrasound contrast agent. When their 3D studies were compared with diagnostic cerebral angiography, they had imaging success rates of 100% for the ipsilateral anterior cerebral artery, middle cerebral artery (with three or more branches), posterior cerebral artery, and posterior communicating artery, and 90% for the anterior communicating artery. On the side contralateral to the transducer, imaging success rates were 90% for the anterior cerebral artery, 80% for the middle cerebral artery (again with three or more branches), and 100% for the posterior cerebral artery. They concluded that 3D transcranial power Doppler angiography with contrast agent enhancement provided significant improvements in the visualization and evaluation of the major intracranial arteries.
Recent Clinical Experience Using Power Doppler Angiography
A number of other reports have been published on the clinical utility of power Doppler ultrasound in vascular patients [27–30]. The most recent report evaluated 53 patients referred to the vascular laboratory who had conventional color duplex ultrasound followed by power Doppler imaging [31]. All 53 patients had conventional arteriography to verify the duplex ultrasound results. Patients were seen for the following indications: Carotid/Vertebral Artery – (1) subtotal versus total carotid occlusion; (2) tortuosity of the artery with limited imaging; (3) the presence of significant disease by spectral Doppler velocity measurements with limited imaging on conventional duplex examination; (4) patients with heavy calcification and limited imaging; and (5) high and/or deep internal carotid arteries. Deep Lying Arteries – Renal/Aorta: (1) limited imaging secondary to obesity or bowel gas and (2) nonvisualization of the renal origin. Peripheral Arteries – (1) subtotal versus total occlusion and (2) anatomy with limited imaging on conventional color duplex ultrasound. The color duplex imaging and the power Doppler examinations were performed by experienced registered vascular technologists in an accredited vascular laboratory using an HDI 5000 Phillips, ATL system (Bothell, WA). Sequential parallel longitudinal views and perpendicular cross-sectional views were obtained on both color duplex and power Doppler imaging. The display quality of both sonographic examinations was classified as satisfactory or unsatisfactory by both the technologist and the interpreting physician. A satisfactory image was defined as one with clearly seen anatomy and well-visualized pathology of the examined vessel. Power Doppler imaging was considered to be of positive diagnostic value if the results of that examination were helpful in differentiating subtotal from total occlusion, optimizing image quality, or visualizing deep-lying vessels (e.g., renal artery) that were not seen on conventional duplex examination. If the two portions of the duplex examination were inconsistent, the final conclusion was decided based on the results obtained by conventional arteriography.
A positive diagnostic value was achieved using power Doppler imaging in 22 out of 29 (76%) carotid artery examinations. Similarly, 10 out of 14 (71%) peripheral artery examinations had a positive diagnostic value. Four out of five (80%) renal artery examinations had a positive diagnostic value, while three out of five (60%) aortoiliac examinations had a positive diagnostic value. Overall, a positive diagnostic value was achieved by adding power Doppler imaging in 39 out of 53 arteries (74%, Table 54.1). The overall sensitivity of power Doppler imaging in patients with a positive diagnostic value was 95% and the positive predictive value was 97%.
Table 54.1
Positive diagnostic value of power Doppler arteriographya
Arteries | Positive Dx value | No change | Total |
|---|---|---|---|
Carotid/vertebral | 22 (76%) | 7 (24%) | 29 |
Peripheral | 10 (71%) | 4 (29%) | 14 |
Renal | 4 (80%) | 1 (20%) | 5 |
Aorta/iliacs | 3 (60%) | 2 (40%) | 5 |
Total | 39 (74%) | 14 (26%) | 53 |
Table 54.2 summarizes the indications of power Doppler imaging and the positive diagnostic value in carotid artery applications. As noted, five out of six patients (83%) who were believed to have total carotid occlusion by conventional color duplex were confirmed to have subtotal occlusion by adding power Doppler imaging. However, 10 out of 14 (71%) carotid artery patients who were believed to have suboptimal images had an improved image on power Doppler imaging. Table 54.3 summarizes the findings in patients for whom the indications included peripheral arteries, renal arteries, and aortoiliac arteries. Six out of eight (75%) patients with a questionable subtotal versus total occlusion by conventional duplex examination were confirmed to have subtotal occlusion by power Doppler imaging. Overall, 10 out of 14 (71%) patients with peripheral arterial examinations had a positive diagnostic value. Four out of five (80%) renal examinations resulted in a positive diagnostic value, which included three patients in whom the origin of the renal arteries was not seen by conventional duplex examinations. Three out of five (60%) aortoiliac examinations resulted in a positive diagnostic value. Figures 54.5, 54.6, 54.7, 54.8, 54.9, 54.10, 54.11, 54.12, and 54.13 illustrate various clinical scenarios where a positive diagnostic value was achieved by adding power Doppler imaging.
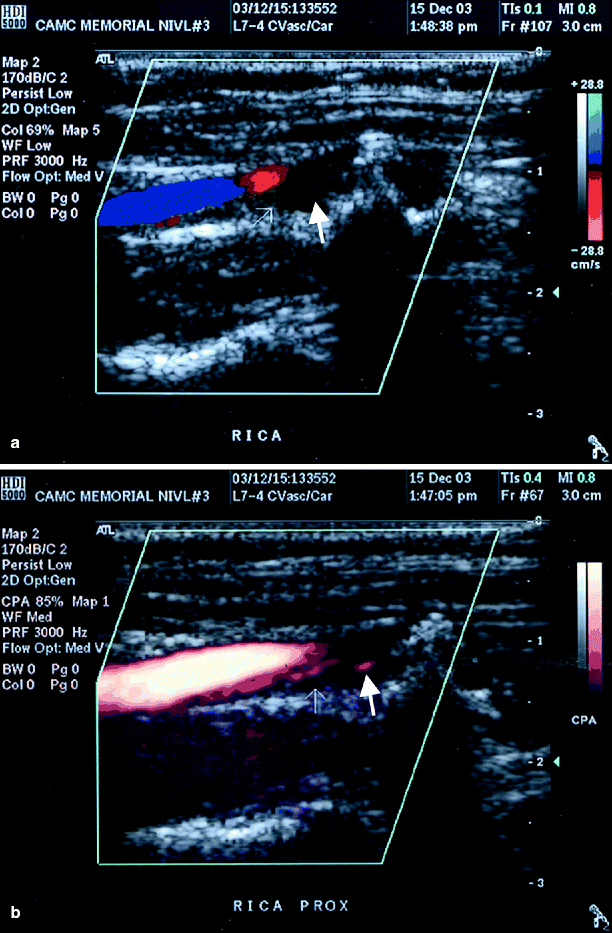
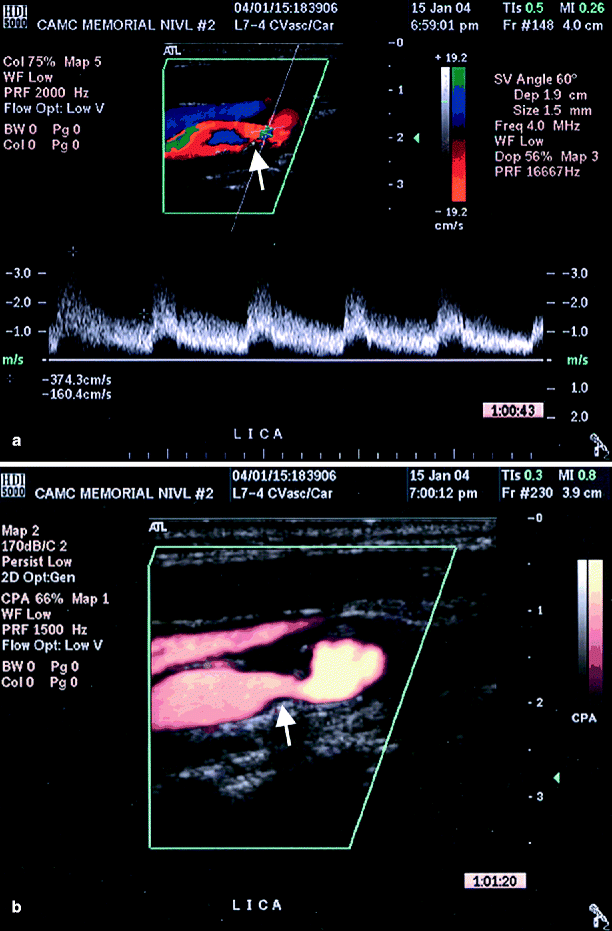
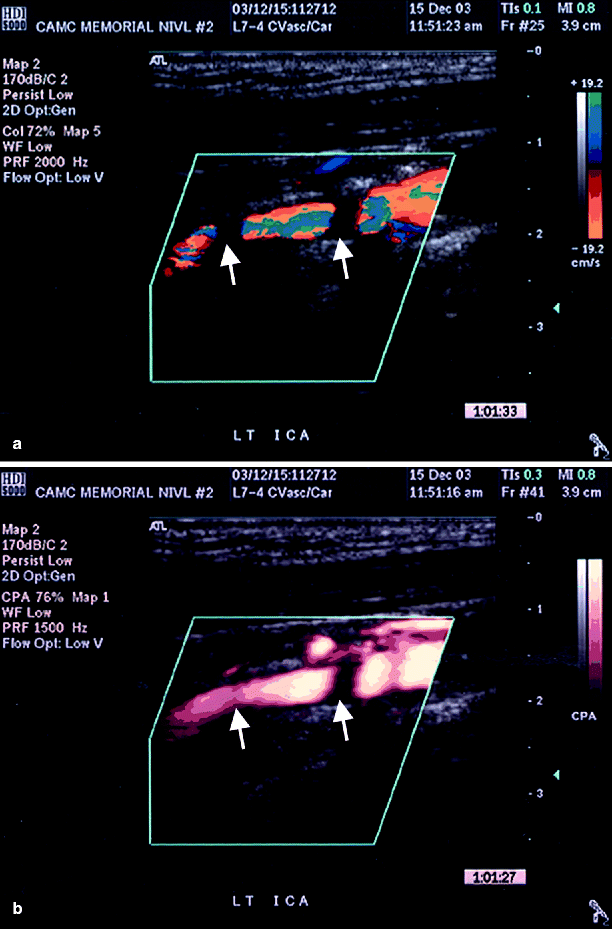
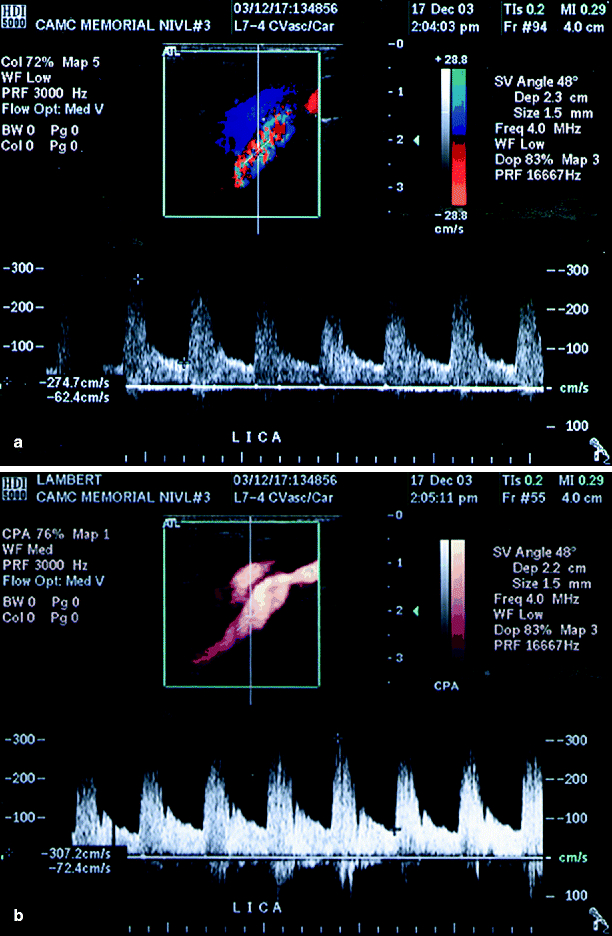
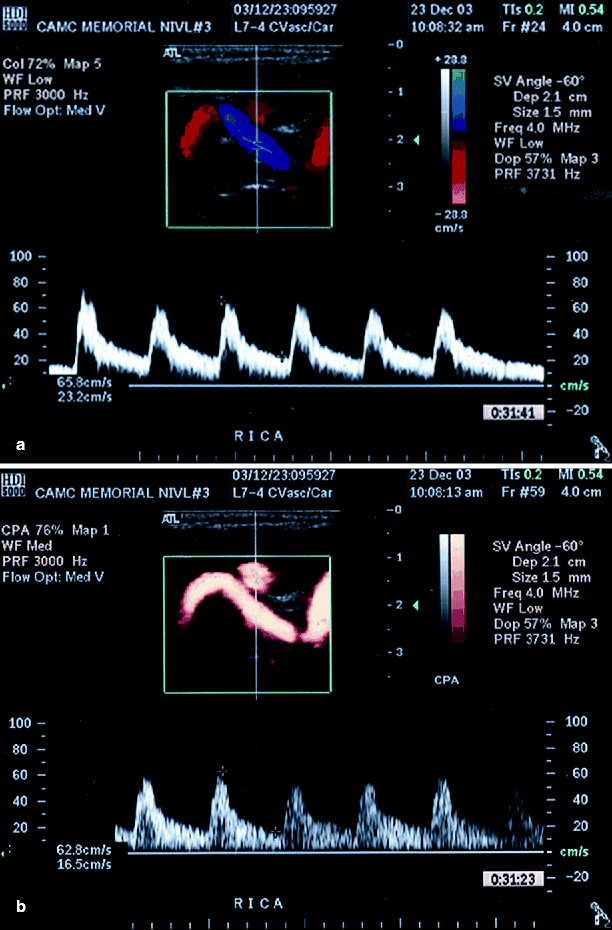
Table 54.2
Carotid indications for power Doppler imaging
Positive Dx value | No change | Total | |
|---|---|---|---|
Subtotal/total occlusion | 5 (83%) | 1 (17%) | 6 |
Suboptimal image | 10 (71%) | 4 (29%) | 14 |
High/deep internal carotid artery | 4 (80%) | 1 (20%) | 5 |
Tortuous artery | 3 (75%) | 1 (25%) | 4 |
Total | 22 (76%) | 7 (24%) | 29 |
Table 54.3
Peripheral and renal indications for power Doppler imaging
Positive Dx value | No change | Total | |
|---|---|---|---|
Peripheral | |||
Subtotal/total occlusion | 6 (75%) | 2 (25%) | 8 |
Suboptimal image | 4 (67%) | 2 (33%) | 6 |
Total | 10 (71%) | 4 (29%) | 14 |
Renal | |||
Obscure origin | 3 (100%) | 0 | 3 |
Suboptimal image | 1 (50%) | 1 (50%) | 2 |
Total | 4 (80%) | 1 (20%) | 5 |
Aorta/iliacs | |||
Suboptimal image | 3 (60%) | 2 (40%) | 5 |

Fig. 54.5
(a) Color duplex imaging of a right ICA suggesting total occlusion (arrows), (b) power Doppler image of same artery showing string sign (arrow), i.e. subtotal occlusion (Reproduced from AbuRahma et al. [31]. With permission from BC Decker, Inc.)

Fig. 54.6
(a) Suboptimal image of left ICA by conventional duplex ultrasound (arrow), (b) better image is obtained by adding power Doppler ultrasound (arrow) (Reproduced from AbuRahma et al. [31]. With permission from BC Decker, Inc.)

Fig. 54.7
(a) Left ICA with multiple defects of color flow (secondary to shadowing, arrows), (b) color flow is seen in one area and somewhat seen in the other area (arrows) when power Doppler was added (Reproduced from AbuRahma et al. [31]. With permission from BC Decker, Inc.)

Fig. 54.8
(a) A deep-lying left ICA with suboptimal imaging on color duplex ultrasound, (b) shows a well-defined stenosis using power Doppler ultrasound (Reproduced from AbuRahma et al. [31]. With permission from BC Decker, Inc.)

Fig. 54.9




(a) A tortuous ICA with poor image of the tortuous segment, (b) clearly shown tortuosity using power Doppler imaging (Reproduced from AbuRahma et al. [31]. With permission from BC Decker, Inc.)
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


