Chapter 20
Venography
David L. Gillespie, Xzabia A. Caliste
Contrast-enhanced venography has been practiced for more than half a century. In 1940, Bauer published details of normal venographic anatomy and the venographic appearance of acute and chronic deep venous thrombosis (DVT).1 Early attempts at venography were marked by complications related to the equipment, contrast agents used at that time, and incomplete understanding of venous hemodynamics. Since 1960, the procedure has become the leading diagnostic test for DVT.2 The technique evolved during many years and became the “gold standard” for the diagnosis of DVT after Rabinov and Paulin published their seminal article on contrast-enhanced venographic technique in 1972.3 Most current techniques are modifications of their approach to venography. Early leg venography required venous cutdown for access to the deep venous system, but this technique was modified by Welch and coworkers, who recommended that contrast material be injected into a superficial vein in the foot with a tourniquet applied above the ankle to prevent filling of the superficial veins, which often obscured the deep system.4
Basic Principles
The two primary techniques of venography that we employ today are ascending and descending venography. Ascending venography is more common and is used to elucidate the presence of DVT in the lower extremity. Descending venography predominantly evaluates incompetent valves in patients with chronic venous insufficiency. This requires direct access to the deep venous system. The techniques of ascending and descending venography are described in the next section on clinical applications.
Clinical Applications
The current indications for venography are detailed in Box 20-1. Despite the availability of less invasive techniques, venography is still considered the gold standard against which all examination modalities are compared to determine the presence of DVT. This is particularly true in instances in which duplex ultrasound is inconclusive or unavailable. Venography also continues to have a higher sensitivity and specificity in detection of infrapopliteal thrombotic disease. Venography is indicated in evaluation of valvular insufficiency and venous malformations, and it has been shown to be helpful in planning adjunctive techniques in the operating room. Relative contraindications to venography are also listed in Box 20-1. Other common modalities used in detection of thrombosis and reflux are compared in Table 20-1.
Table 20-1
Comparison of Various Modalities for the Detection of Venous Disease
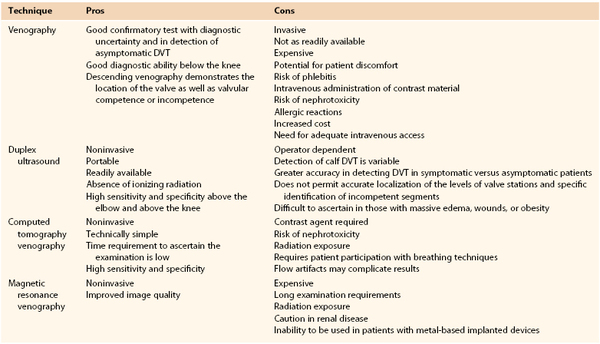
DVT, Deep venous thrombosis.
Ascending Venography
Ascending venography has been used for many indications, including DVT, incompetent perforating veins, and venous aneurysms and malformations.
Deep Venous Thrombosis
DVT cannot be reliably diagnosed by clinical examination. Indications for ascending leg venography include a nondiagnostic ultrasound study and a need for better imaging of calf veins in the setting of high clinical suspicion of DVT.5 Because of its high diagnostic accuracy, a leg venogram is immediately useful for evaluation of the presence of deep venous disease. Another indication is delineation of DVT before endovascular intervention, such as thrombolysis, thrombectomy, or angioplasty.
There are a number of problems with venography, such as inability to cannulate the appropriate vein because of edema, incomplete venous filling, and other technical issues, all of which lead to inadequate studies in as many as 5% to 15% of cases.6,7
Technique.
Ascending venography requires a 22-gauge catheter, three 25-mL syringes, two tourniquets, a tilting fluoroscopic table with footrest, C-arm fluoroscopy, contrast agent, and normal saline. In addition, a 5F sheath should be available for direct access to the venous system through the popliteal vein if needed. It begins with placement of a catheter in a peripheral vein. The access device is usually a 22-gauge intravenous catheter inserted into a superficial vein on the dorsum of the foot. When the affected extremity has significant edema, percutaneous catheterization of peripheral veins becomes difficult. In such cases, we use ultrasound to guide vascular access. It is important that access for ascending venography not be through the saphenous vein adjacent to the medial malleolus at the ankle because contrast material may preferentially fill the superficial system without demonstrating the deep venous systems adequately. The likelihood of this problem can be decreased by placing a tourniquet at the ankle and one at the knee, which will drive the contrast material into the deep venous system. However, these tourniquets should be released just before image acquisition to relieve extrinsic compression and to avoid occlusion of the deep veins.
The patient is placed on a tilting fluoroscopic table and a footrest to enable near-upright positioning. With the patient initially in a reverse Trendelenburg position, the table is tilted to recumbency as the bolus is tracked. This approach enhances visualization of the large-capacity lower extremity venous anatomy as gravity delays the outflow of contrast material. The contralateral leg is supported with a small platform so that no weight is borne on the leg to be examined. Side grip handles can be placed on the table as needed, depending on the patient’s comfort and security in the semiupright position.
The examination is begun with the patient in a 40- to 60-degree semiupright position. As contrast material is injected, fluoroscopic evaluation is performed to ensure that there has been no extravasation of contrast and to optimize deep vein opacification. The bolus is followed by radiographic imaging as the material flows to the central veins. As the contrast agent ascends, additional filming of the deep and superficial femoral veins is performed. At least two projections are needed in the tibial and popliteal locations. About 50 to 100 mL of contrast material is needed to fill the deep venous system adequately from ankle to groin. Additional contrast material can be injected, as needed, to visualize problem areas.
Even with appropriate venous access and the use of tourniquets, adequate opacification of the deep veins of the lower extremities may be difficult because of dilution of the contrast agent and preferential flow from deep to superficial regions, which allows contrast material to escape to the superficial system. In such cases, we place the patient in the prone position, access the popliteal vein under ultrasound guidance, insert a 5F sheath, and inject contrast material into the deep venous system. At completion of the examination, the veins are flushed with 50 mL of normal saline to minimize contact of the contrast agent with the venous endothelium and the chance for development of thrombophlebitis.
Interpretation.
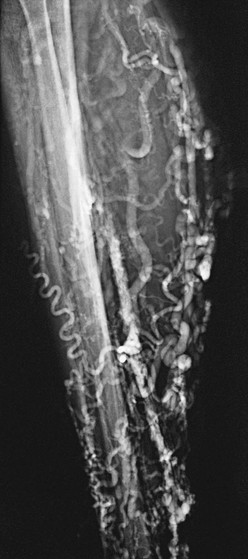
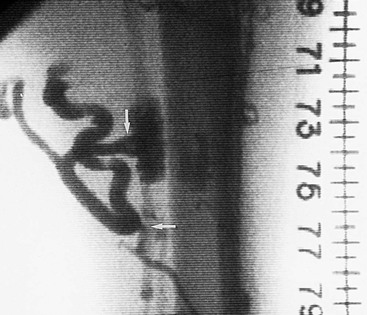
The classic venographic sign of venous thrombosis is a luminal filling defect with a surrounding rim of contrast (Figs. 20-1 to 20-6). This appearance of parallel lines of contrast material around the thrombus is referred to as the “tram-track” sign. Other indicators of thromboembolism are an abrupt termination of intravascular contrast or the formation of a meniscus. Correct and consistent interpretation of lower extremity venography to rule out DVT, however, may be difficult. Intraobserver disagreement about the probable presence or absence of thrombus has been shown to occur in up to 10% of venographic cases.8 The invasive nature of the technique, the exposure to radiation, and the upsurge of ultrasound technology have made venography a secondary technique for the detection of acute and chronic venous thrombosis.
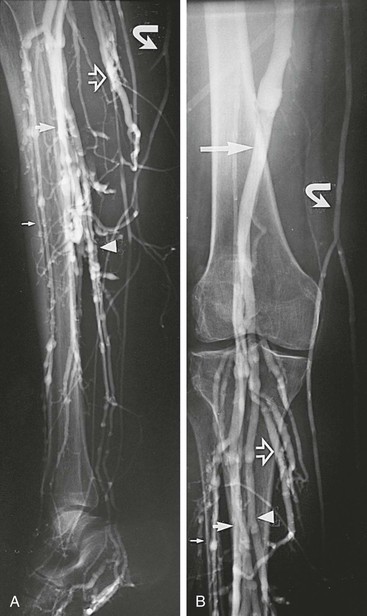
Figure 20-1 Normal right leg venogram. A, Lateral calf projection. B, Anteroposterior projection of the knee. The anterior tibial veins (small arrow), peroneal veins (short arrow), posterior tibial veins (arrowhead), gastrocnemius veins (open arrow), great saphenous vein (curved arrow), and popliteal vein (long arrow) are indicated.
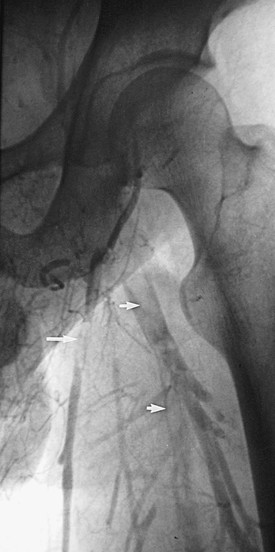
Figure 20-2 Acute thrombus in the deep femoral and great saphenous veins. The deep femoral vein (short arrows) and the great saphenous vein (long arrow) are shown. The femoral vein is occluded.
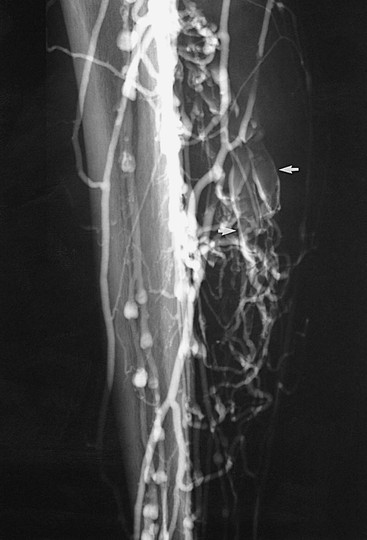
Figure 20-3 Acute thrombosis of the soleal veins. Arrows indicate fresh thrombus filling the soleal veins.
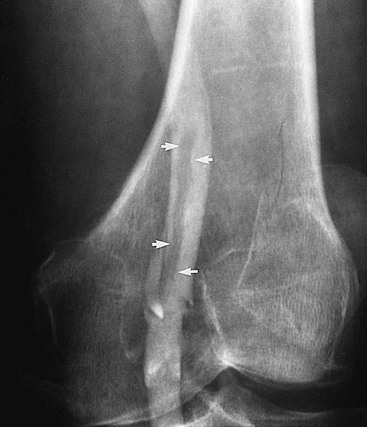
Figure 20-4 Subacute thrombus in the popliteal vein. Arrows denote retracted thrombus, indicative of subacute thrombosis.
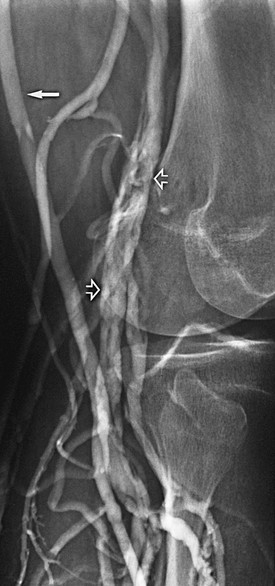
Figure 20-5 Chronic popliteal venous thrombosis. Note the extensive recanalization of the popliteal vein (open arrows) and relatively large caliber of the great saphenous vein (solid arrow).
Incompetent Perforating Veins
The normal outflow from veins of the lower extremity includes pathways from the superficial system to the deep system through perforating veins. Perforating veins possess unidirectional valves, which, when competent, prevent reflux of blood from the deep into the superficial veins of the leg. Perforating veins with incompetent valves contribute to the development of venous hypertension in the superficial system, which results in the formation of varicose veins and venous ulceration.
Normal perforating veins are small, thin, and smooth. Incompetent veins, however, are dilated, irregular, and valveless. Historically, ascending venography has been a reliable technique for identifying incompetent perforating veins. This invasive technique, although useful, has largely been replaced by color-flow duplex examination of the lower extremity.
Technique.
Ascending venography for incompetent perforating veins involves the injection of contrast material into a foot vein with a tight tourniquet around the ankle to occlude the superficial veins such that reverse flow from the deep veins into the superficial veins through the incompetent perforating veins can be seen. A translucent ruler is then used to measure the location of the incompetent perforating vein from the tip of the medial or lateral malleolus. The table is next moved through the horizontal position and then into Trendelenburg position, and flow of contrast material is followed under fluoroscopy. Lateral views are especially important in the thigh for accurate localization of a midthigh perforating vein. Once a perforating vein is identified, a tourniquet can be used above that level to direct the contrast agent into any incompetent perforating vein.
Interpretation.
Thomas and Bowles compared ascending venography with varicography for identification of incompetent perforating veins.9 Sixty-one legs of 50 patients were examined with both methods. Incompetent perforating veins of the gastrocnemius muscle and midthigh were more accurately shown with varicography than with ascending venography (Fig. 20-7).
Venous Aneurysms and Malformations
The most serious complication associated with lower extremity venous aneurysms is the development of thrombus within the aneurysm and subsequent embolization. Pulmonary embolization has been reported to occur frequently in extremity venous aneurysms, most notably in those involving the popliteal vein (Fig. 20-8). Aldridge and coauthors in 1993 reported 24 cases of popliteal venous aneurysm in which all patients had experienced thromboembolic events.10 Aggressive treatment with immediate anticoagulation and preoperative planning has become the standard of care for this reason. Venography is a useful method for preoperative planning before resection and interposition grafting or lateral suture repair of these aneurysms.
Venous malformations may take the form of isolated venous lakes or diffuse venous aneurysm formation, such as Klippel-Trénaunay syndrome (Fig. 20-9). Patients often become symptomatic from mass effect, reflux, or venous thromboembolism. Operative or interventional management may be indicated in symptomatic patients. Most patients do well without treatment or with elastic compression only. Surgical treatment of the vascular malformation in Klippel-Trénaunay syndrome is rarely needed and continues to be controversial.11 Preoperative venous imaging with ascending venography or direct stick varicography is useful for planning treatment.
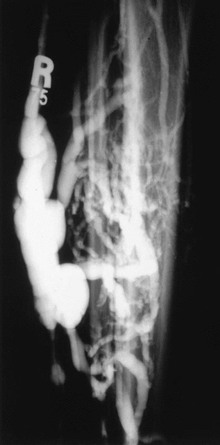
Figure 20-9 Tibial venous malformation in a patient with Klippel-Trénaunay syndrome. (From Gillespie DL, et al: Presentation and management of venous aneurysms. J Vasc Surg 26:845-852, 1997.)
Technique.
The technique used is the same as when ascending venography is performed. Direct stick varicography is most useful for the evaluation of venous malformations (see Figs. 20-8 and 20-9). Ultrasound-guided access provides the ability to perform direct stick venography. In patients being treated for venous malformations, access can be difficult and painful. In such cases, either general or regional anesthesia may be useful.
Interpretation.
Focal dilatation of the deep or superficial venous system is diagnostic of venous aneurysms and malformations (see Figs. 20-8 and 20-9). Because of the small number of venous aneurysms included in most individual series, no single diagnostic method has been reported to be superior to another. Most series, however, do report color-flow duplex examination to be extremely valuable in the diagnosis of popliteal venous aneurysms and to provide information on the presence of mural thrombus.12 Gillespie and associates found in their series that venous aneurysms were correctly diagnosed in 85% of patients with imaging techniques: phlebography (60%), color-flow duplex scanning (27%), and magnetic resonance imaging (10%).13
Passive filling of the vascular tree after compressive exsanguination of an extremity, extrinsic occlusion of its arterial supply, and venous drainage has been shown to be a useful technique for evaluation of venous malformations.14 Closed-system venography was performed in 17 patients, and it correctly identified 11 of 12 surgically confirmed vascular abnormalities. There were no false-positive findings.
Descending Venography
As with ascending venography, lower extremity descending venography is no longer used as a screening study. It has largely been replaced by color-flow duplex study, which has shown good agreement with descending venography in the grading of deep and superficial vein reflux.15 However, duplex examination is limited in that it does not permit accurate localization of the levels of valve stations and specific identification of incompetent segments. For this reason, descending venography remains the definitive test for identifying incompetent valves in patients with chronic venous insufficiency who are candidates for venous valve repair or valve transplantation.
Descending venography is used for evaluation of the anatomy and function of the venous valves in the lower extremities. It shows the location of the valves in the veins and also demonstrates the competence or incompetence of these valves. Kistner16 and Herman17 and colleagues developed a classification system used commonly to categorize the severity of deep venous reflux and the functional integrity of the venous valves (Table 20-2).
Table 20-2
Grades of Venous Reflux
| Grade | Description |
| 0 | Normal valvular function with no reflux |
| 1 | Minimal reflux confined to the upper part of the thigh |
| 2 | More extensive reflux, which may reach the lower part of the thigh; a competent valve is present in the popliteal vein, and there is no reflux to the calf level |
| 3 | Reflux as above but associated with popliteal valvular incompetence and leakage of contrast material into the calf veins |
| 4 | Virtually no valvular competence with immediate and dramatic reflux distally into the calf; this type of reflux often opacifies incompetent calf perforating veins |
From Kistner RL, et al: A method of performing descending venography. J Vasc Surg 4:464-468, 1986; and Herman RJ, et al: Descending venography: a method of evaluating lower extremity venous valvular function. Radiology 137:63-69, 1980.
Technique.
Descending venography requires a tilt radiographic table, C-arm fluoroscopy, a short 4F or 5F straight catheter with distal side holes, syringes, and contrast agent. It is performed on a tilt radiographic table to allow examination in the 40- to 60-degree upright position. Descending venography always requires direct catheter access into the deep veins. In most cases, the venous access site is the common femoral vein contralateral to the side of interest. The catheter is advanced to the inferior vena cava (IVC) and then to the iliac vein and common femoral vein of the symptomatic lower extremity. A short 4F or 5F straight catheter with distal side holes is positioned at the junction of the external iliac vein and common femoral vein (Fig. 20-10). For unilateral examination, the catheter is placed from the ipsilateral femoral vein, and for bilateral examination, a single femoral vein puncture is used. A transjugular approach may be used to direct the retrograde catheter into both lower extremities from a single access site. The contralateral leg is supported with a small platform, as with ascending venography, so that the leg being examined will be relaxed and bear no weight.
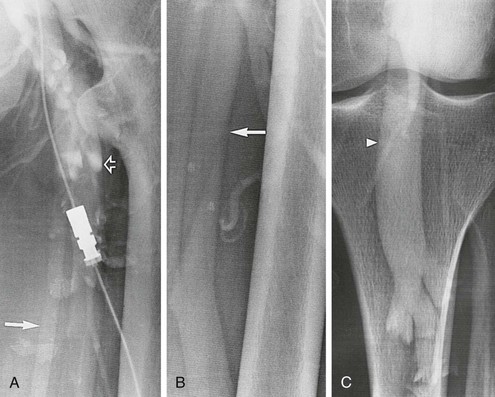
Figure 20-10 A-C, Descending left leg venogram showing grade 4 reflux. Note the lack of valves in the femoral vein (solid arrow) and the large caliber of the popliteal vein (arrowhead). The deep femoral vein (open arrow) is shown.
With the catheter tip in the common femoral vein, the table tilted to a 60-degree semiupright position, and the patient bearing weight on a block placed under the nonexamined leg, contrast material is injected and tracked by fluoroscopy as it flows caudad through the incompetent venous valves. A total of 10 mL of contrast material is slowly injected by hand at a rate of 5 mL/s. As the contrast material is injected, the patient breathes normally, after which the patient is instructed to bear down and perform a Valsalva maneuver to enhance evidence of valvular incompetence. Specific areas of interest can be further studied by sequential injections of contrast material to optimize image capture of valve location and function and the extent of reflux.
Interpretation.
Although superficial varicosities are frequently present, they are rarely a prominent feature of symptomatic advanced chronic venous disease.18 Most patients with symptoms of chronic venous insufficiency who undergo venography have reflux into the deep venous system. The two main abnormalities that cause venous valve reflux are postphlebitic valvular incompetence and primary valvular incompetence, with primary valvular incompetence being responsible more frequently.19 With the use of descending venography, candidates are selected for deep venous valve repair or transplantation, which is offered to patients with grade 3 or grade 4 reflux who have recurrent symptoms of venous insufficiency after treatment of superficial varicosities and perforating vein incompetence (see Fig. 20-10 and Table 20-2).
Iliac Vein Assessment and Inferior Venacavography
IVC anatomy and patency are routinely assessed as part of IVC filter placement. Phlebography allows assessment of caval anatomy and evaluation for the presence of caval thrombus. In addition, there are several anomalies of the IVC, as outlined in previous chapters. Duplicated cavae, megacavae, and left-sided venae cavae are but a few of these anomalies. Although these anomalies are uncommon, it is important to routinely assess the vena cava with venography before placement of IVC filters to avoid complications caused by either filter misplacement or leaving the patient vulnerable to pulmonary embolism through alternative pathways.
Technique
Access to the venous system is achieved through the internal jugular or common femoral vein. Puncture of just a single wall is facilitated by ultrasound guidance, and a micropuncture kit is used to minimize access size. Once access is achieved, a 5F sheath is placed and proper placement confirmed by free flow of venous blood from the sheath.
From the groin, a 0.035-inch J wire is passed into the common iliac vein. A 5F pigtail catheter is advanced over this wire into the common iliac vein, and cavography is performed.
An alternative method of cavography is to proceed with placement of the IVC filter kit, which consists of a sheath, usually 8F. The insertion track is serially dilated from 5F to 8F. The 8F sheath is advanced over the wire to the level of the common iliac vein, and a power-injected cavogram is performed.
From the neck, a 0.035-inch J wire is passed through the right atrium to the common iliac vein. Passing the J wire through the right atrium into the IVC can be somewhat of a challenge, however. If simple manipulation fails, a deflecting wire can be useful. Once the J-wire is in position, an iliocavogram is performed using a protocol of 10 mL/s for a total of 20 mL of nonionic contrast agent.
After completion of the iliocavogram, the pigtail catheter is removed over a wire. All wires are then removed and the sheath is pulled. Hemostasis should be obtained easily with digital compression for 10 to 15 minutes, depending on whether anticoagulation has been administered.
Interpretation
The iliocavogram is reviewed for the diameter of the vena cava, the anatomic level of the bifurcation, the level of the renal veins, and any other anomalies (Figs. 20-11 and 20-12). Typically, the renal veins will not fill with contrast material and instead will be identified by a characteristic flow pattern of the mixing of blood filled with contrast material and unopacified blood. The confluence of the common iliac veins is usually identified by the spilling over of contrast material into the contralateral side.
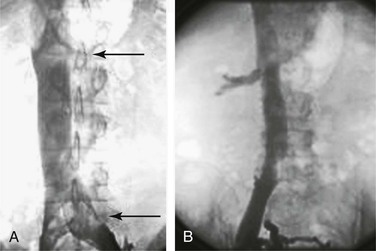
Figure 20-11 A, Inferior venacavogram demonstrating confluence of the common iliac veins and a normal flow pattern formed by mixing of unopacified renal vein blood with contrast material in the inferior vena cava (arrows). B, Inferior venacavogram demonstrating filling of the renal veins.
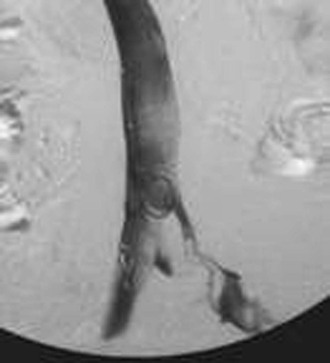
Figure 20-12 Inferior venacavogram demonstrating left common iliac vein deep venous thrombosis (unknown source).
Some centers advocate first-order selective venography instead of or as a supplement to nonselective venography. Danetz and coworkers found that when selective venography was used in conjunction with nonselective venography, 23% of patients had either an abnormal finding or aberrant anatomy, and most of these patients required a major change in vena cava filter position.20
Before removal of the IVC filter, the surgeon should examine whether the filter has retained thrombus, penetrated the IVC, migrated, or angulated (Fig. 20-13). Severely angulated temporary filters with more than 20 degrees of tilt are more likely to make removal unsuccessful.21
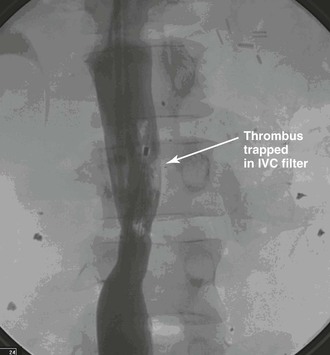
Figure 20-13 Venogram of the inferior vena cava (IVC) before attempted retrieval of a prophylactic IVC filter with trapped thrombus (arrow). (Courtesy Eric Adams, MD.)
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree