Chapter 53
Vena Cava Interruption and Pulmonary Embolism
Marc A. Passman
Venous thromboembolism (VTE) is a significant cause of morbidity and mortality that affects approximately 1 per 1000 of the population, with an annual estimate of 600,000 cases of clinically significant pulmonary embolism (PE) and about 200,000 deaths in the United States.1,2 For most situations, VTE is effectively managed with anticoagulation, which is associated with decreases in recurrence and PE, lower mortality, and an acceptably low risk of bleeding complications. However, when anticoagulation is ineffective or contraindicated or results in complications requiring it to be discontinued, vena cava interruption with a filter device is recommended.
With the understanding that lower extremity deep venous thrombosis (DVT) can fragment and travel through the inferior vena cava (IVC) to the lung, the concept of vena cava interruption has been around since the 1700s. In 1784, John Hunter performed the first femoral vein ligation for thrombophlebitis, and in 1893, Bottini reported successful ligation of the IVC to prevent PE. Since then, numerous techniques of vena cava interruption have been described for the prevention of PE, including femoral vein and IVC ligation and partial interruption of the IVC by means of plastic clips, plication, or mechanical staplers.3–6 These later measures were developed to compartmentalize the vena cava to allow blood flow but trap large emboli, and they were the mainstay of treatment through the 1950s and 1960s. Despite these measures, there were high rates of IVC occlusion and significant lower extremity vein–related problems with little improvement in outcome for patients with PE. With the development of techniques to allow transvenous delivery of intravascular vena cava devices, in 1967 the Mobin-Uddin umbrella filter was introduced. Structured as a silicone membrane with a hole to allow blood flow, it was associated with a high rate of vena cava thrombosis and was subsequently discontinued.
In 1973, the original stainless steel Greenfield filter (Boston Scientific, Inc., Natick, Mass) was introduced, followed by titanium Greenfield filter in 1989 and the lower-profile stainless steel Greenfield filter in 1995; as the most extensively studied filter, the Greenfield is the filter design with which all modern filters are compared. Over the past few decades, several additional filter designs have been developed that have favorable properties, including ease of deployment, clot-trapping effectiveness, preservation of IVC flow, and, in later years, optional filter designs that allow retrieval of the filter. Although the original transvenous filters were placed in the operating room through open venous access, advances in technology and lower-profile delivery systems have allowed percutaneous placement in the endovascular suite to become the accepted standard technique, and later evolution has allowed bedside placement of filters under transabdominal or intravascular ultrasound guidance. This chapter reviews filter designs for currently available vena cava filters; clinical indications for filter placement; techniques for placement in the IVC, including venography, transabdominal duplex ultrasound, and intravascular ultrasound; techniques for placement in the superior vena cava; and PE diagnosis and management.
Filter Design
Design Considerations
The functional goal of filters is to prevent PE by trapping venous thromboemboli in the vena cava, not to prevent or treat venous clots. On the basis of this design goal, several features are preferred for optimal function and minimal compromise, including the use of nonthrombogenic, biocompatible, nonferromagnetic, durable, implantable material. Filtering efficiency should be high without impedance of flow, and there should be a single trapping level and conical design, which provides the highest filtering-to–flow volume ratio (Fig. 53-1). Ideally, the filter should have a self-centering mechanism with secure fixation to the vena cava wall, a filter hook length and angle sufficient to prevent migration when the filter is in place, and the ability to be retrieved when no longer needed without limitation of the time frame for incorporation. The optimal filter should be highly visible on imaging with ease of percutaneous delivery through a low-profile system and a simple release mechanism. Repositioning and retrievability should also be possible, and the potential for complications should be negligible. The financial cost of filter delivery should be low.
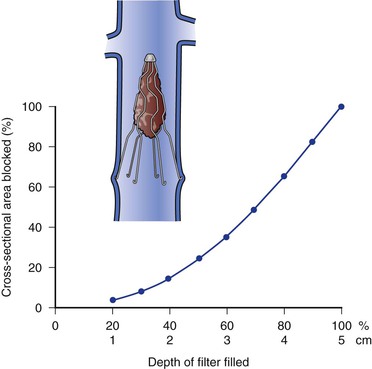
Figure 53-1 Relationship between the percentage and depth of thrombus trapped within the filter and the percentage of cross-section occluded.
Vena cava filter design categories include the following:
• Optional/retrievable filter: Similar to a conventional permanent filter, the optional or retrievable filter has the added feature of removal capability. A retrievable filter adheres to the wall of the vena cava with hooks, barbs, or radial force (or any combination of the three) but can be retrieved by image-guided catheter techniques within a device-specific time interval.7
Individual Filters
Understanding individual filter designs, advantages, and limitations is important before placement to ensure proper filter selection. For the purpose of this chapter, only permanent and optional/retrievable filter designs approved by the U.S. Food and Drug Administration (FDA) are discussed (Table 53-1 and Fig. 53-2). None of the currently available filters has all the desirable attributes of the perfect filter previously listed. Overall, these features are weighted differently in current filter designs, with certain characteristics more important than others, depending on the intended priorities of a filter for certain clinical situations.
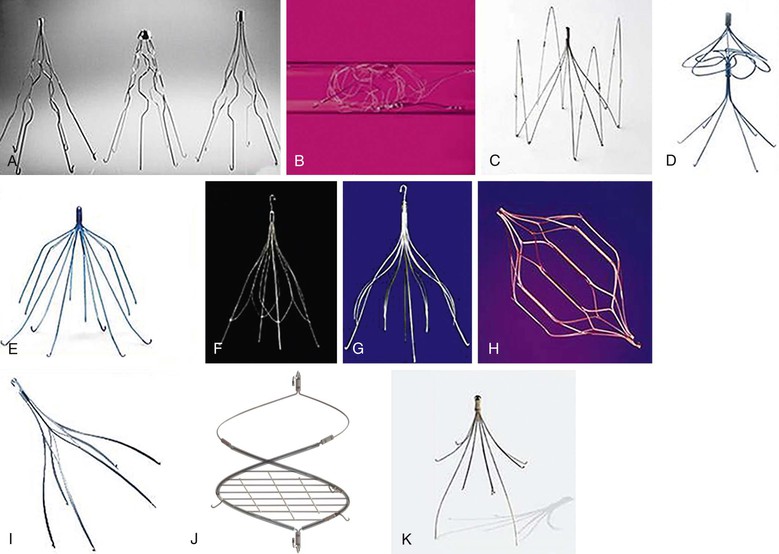
Figure 53-2 Vena cava filter designs currently approved by the U.S. Food and Drug Administration. A, From left to right, Boston Scientific titanium Greenfield filter, original stainless steel Greenfield filter, and low-profile stainless steel Greenfield filter; B, Cook Medical Gianturco-Roehm Bird’s Nest filter; C, Vena Tech LP filter; D, Bard Peripheral Vascular/Simon Nitinol filter; E, Bard Peripheral Vascular Recovery G-2 filter; F, Cook Medical Günther Tulip filter; G, Cook Celect filter; H, Cordis Corporation OPTEASE filter; I, Argon Medical Devices Option filter; J, Crux VCF (Volcano Corporation, San Diego, Calif); K, ALN International Inc Optional filter. Not pictured, Bard Peripheral Vascular Eclipse filter and Bard Peripheral Vascular Meridian filter.
Conical filter design is most optimal and permits progressive central filling while allowing circumferential blood flow peripherally, helping maintain vena cava patency. As an example, the conical geometry of the Greenfield filter makes it possible for thrombi to fill and occlude 70% of the filter cone, a volume of thrombus of approximately 4 cm3 (34.3% of the total volume), while reducing the cross-sectional area by less than 50%. If the cone is filled to 80% of total volume, the reduction in cross-sectional area is 64%, at which point venous flow begins to decrease and venous pressure proximal to the filter begins to increase.8 The ability of this conical filter design to trap clinically significant emboli without impeding flow has been demonstrated. Although other filter designs have double trapping levels or double-basket designs and have higher capacity to capture smaller emboli, their flow dynamics are reduced in comparison with those of single-trapping conical designs and may be associated with higher vena cava occlusion rates.
In addition, although rapid and stable incorporation may be desirable for permanent filter design to maintain position and prevent migration, retrievable filter designs need to have sufficient incorporation to prevent migration but not so much that retrieval cannot be accomplished. Altering the filter hook contact point to allow retrievability may have the disadvantage of a greater tendency for filter leg penetration or filter migration. This change has also resulted in different filter hook lengths and angle configurations. Although some filters, such as the Greenfield filter, incorporate a recurved configuration to create a contact angle of 80 degrees, allowing better hook incorporation without full penetration into the vena cava, other filters use a j-hook configuration to prevent excessive incorporation and facilitate retrievability, although this design change has engendered additional issues with increased vena cava wall penetration or migration. Even newer filter designs, such as the Crux filter (Crux Biomedical, Inc., Menlo Park, Calif), do not depend on filter legs for fixation, thereby eliminating the potential for penetration. Similarly, although some filters do not have self-centering features, others have included independent self-centering mechanisms. To improve self-centering of the filter, some designs have more contact points with the vena cava wall, a feature that prevents tilt but may lead to greater incorporation and a possible tendency for vena cava occlusion. These additional filter features do not offer any additional filtering capacity, may change flow dynamics, and can potentially lead to penetration of the vena cava wall by the filter-centering strut. In the process of choosing the best filter option for the individual clinical scenario, these design characteristics and their risk and benefits should be considered (see Table 53-1).
Clinical Indications
Recommended indications for the use of vena cava filters are shown in Box 53-1. Systemic anticoagulation is the therapy of choice for venous thromboembolism. However, anticoagulation may be contraindicated in some patients. Without anticoagulation, the risk that PE will develop in patients with venous thromboembolism is high, and PE may be fatal in as many as 25% of patients. Because of this significant risk, vena cava interruption with an implantable filtering device should be considered in patients with documented venous thromboembolism and contraindications to anticoagulation. Resumption of anticoagulation as soon as possible is recommended because, although vena cava filters are effective in preventing PE, they are not effective for prevention of DVT.
Evidence-based guidelines from the 2012 American College of Chest Physicians (ACCP)9 recommend vena cava filter placement in the patient with documented venous thromboembolism and a contraindication to anticoagulation, a complication of anticoagulation, or recurrent venous thromboembolism despite therapeutic anticoagulation. Contraindications to or complications of anticoagulation can include need for major surgery, intracranial hemorrhage, pelvic or retroperitoneal hematoma, ocular injury, solid intra-abdominal organ injury, uncorrected major coagulopathy, coagulation disorder, peptic ulcer disease, and other associated medical problems.
Beyond these strong evidence-based guidelines, expanded relative indications based on inconclusive evidence have included poor compliance with anticoagulation; free-floating iliocaval thrombus; renal cell carcinoma with renal vein extension; placement in conjunction with venous thrombolysis or thromboembolectomy; presence of DVT and limited cardiopulmonary reserve or chronic obstructive pulmonary disease; recurrent PE complicated by pulmonary hypertension; proven DVT in a patient with cancer or burn(s) or who is pregnant; and venous thromboembolism prophylaxis in patients with trauma or who are undergoing surgery or medical treatment and have high risk for venous thromboembolism. The venous thromboprophylaxis category is the most controversial because strong level I evidence from randomized trials are still lacking. A clinical decision algorithm for vena cava filter use for prophylaxis of venous thromboembolism is shown in Box 53-2. Recommendations should be based on a combination of high-risk patient factors or situations in which a high risk of bleeding prohibits use of anticoagulation for prophylaxis.
Permanent versus Optional Filter
Filter use has expanded significantly in the United States over the past few decades, especially for prophylaxis of venous thromboembolism.10 Unfortunately, evidence-based recommendations for vena cava filter use are derived predominantly from data from nonrandomized studies, with substantial differences existing among studies in terms of study populations, immediate and long-term end points, and duration of follow-up. Because of difficulties comparing data on different filters, several guidelines outlining reporting standards for filter devices have been published.11–13 Overall, reported complications of the use of vena cava filters include PE (2%-5%), fatal PE (0.7%), death linked to filter insertion (0.12%), venous access site thrombosis (2%-28%), filter migration (3%-69%), vena cava penetration (9%-24%), vena cava obstruction (6%-30%), venous insufficiency (5%-59%), filter fracture (1%), and guide wire entrapment (<1%).14,15
Although it is unclear whether filters actually improve survival, they do provide protection against PE. Because the risk for PE in patients with proven venous thromboembolism and contraindications to anticoagulation is high, potential complications of filters must be balanced against the risk of no filter. Despite the large number of filters placed, only one randomized study on permanent filter use has been conducted, and it showed some correlation with increased potential problems with permanent filters over time. In the PREPIC (Prevention of Recurrent Pulmonary Embolism by Vena Cava Interruption) trial, 400 patients with proximal DVT with or without PE were randomly allocated in a 2 × 2 factorial study design to receive filter placement versus no filter and unfractionated heparin versus enoxaparin. There was a significantly lower incidence of PE with filter protection during the first 12 days (1.1% vs. 4.8%), but the filter group had a significantly higher incidence of recurrent DVT at 2 years (20.8% vs. 11.6%).16 Eight-year follow-up data from the PREPIC trial confirmed the previous findings of cumulative recurrent PE (6.2% filter vs. 15.1% no filter) but increased recurrent DVT (35.7% vs. 27.5%, respectively) and no difference in postthrombotic venous insufficiency or survival.17 The investigators concluded that although the use of permanent filters may be beneficial in patients at high risk for PE, systematic use of such filters in the general population with venous thromboembolism is not recommended. Unfortunately, no firm conclusions regarding filter efficacy in the prevention of PE can be drawn from the PREPIC trial, given that the study design, which used anticoagulation, varied significantly from the wider application of permanent filters in current clinical practice—notably, in patients with documented venous thromboembolism in whom anticoagulation has failed or cannot be administered.
Although the potential for significant adverse outcomes with permanent filters is low, there are long-term complications that could be avoided with optional filters. However, given the paucity of data from randomized studies, determining the best permanent versus optional filter design is difficult. Some studies have used meta-analysis of data to compare the different filter designs and document the efficacy of filters in the prevention of PE and complication rates.18–20 In one systematic review of published literature, retrievable IVC filters placed in high-risk patients for whom anticoagulation was not feasible seemed to be effective in preventing PE, but concern was raised regarding long-term complications with the use of these filters, although the evidence of the effectiveness and of the risks was limited by the small number of prospective studies. Table 53-2 summarizes data on different filter types; overall, most of the available filters are roughly equivalent in prevention of PE, but there is some variation in complication rates.21,22 Confounding any comparison is the fact that studies of the more recently approved filters, which appear to have lower PE rates, have smaller study populations and shorter follow-up intervals, thus making any comparative analysis imprecise.
Data on the efficacy and safety of retrievable filters are derived from small series with insufficient long-term data in comparison with other permanent filter designs. With the lack of extended outcome data for optional filters, the decision to use a retrievable filter instead of a permanent filter should be based on the intent to discontinue filtration. Factored into this decision should be the anticipated required duration of protection from venous thromboembolism versus the risk associated with anticoagulation. In patients with proven venous thromboembolism, anticoagulation should be resumed as soon as possible when the risk has diminished. For patients in whom filters are placed for prophylaxis of venous thromboembolism, anticoagulation should be restarted in accordance with published venous thromboembolism prophylaxis guidelines. When the risk of resuming anticoagulation is extended, permanent filtration would be preferable.
Further complicating the decision to use retrievable filters is poorly defined timing of possible retrieval. On the basis of FDA indications for use (IFU), retrieval of Günther Tulip filters (Cook Medical, Inc., Bloomington, Ind) is recommended within 20 days and of OPTEASE filters (Cordis Corporation, Bridgewater, NJ) within 14 days. The Recovery G-2 filter (Bard Peripheral Vascular, Tempe, Ariz) has a recommended retrieval window within a mean of 140 days and a maximum of 300 days, and the Celect filter (Cook Medical, Inc.), within 52 weeks. More recently approved filters—like the G-2 Express, Eclipse, Meridian, and Denali (all Bard Peripheral Vascular), Option (Argon Medical Devices, Inc., Plano, Tex), ALN Optional (ALN, Bormes-les-Mimosas, France), and Crux—do not have clearly specified time windows according to FDA indications for use. Further confusing optimal timing are case reports suggesting that potential retrieval at extended periods is possible for all filters but can be more problematic with a higher failure rate.23–26 Regardless, the longer a filter is in place, the less successful its retrieval will be, as shown in a systematic review showing technically successful retrieval rates of 99% at 1 month and 94% at 3 months but dropping to 37% at 12 months.27 Until further data are available, the optimal period for retrieval of currently available optional filters probably falls within a few months of placement, after which the chances for technical success of retrieval will diminish.
Although optimal timing and technical feasibility of retrieval are suggested by the FDA indications for use for each filter, clinical decision making should also factor in the risk of leaving retrievable filters in place versus a diminishing risk of PE. With the expanding use of retrievable vena cava filters, a growing body of literature and reports to the FDA’s Manufacturer and User Facility Device Experience (MAUDE) database led the FDA in 2010 to issue a communication about the risk of adverse events with the long-term use of such filters: “Since 2005, the FDA has received 921 device adverse event reports involving IVC filters, of which 328 involved device migration, 146 involved embolizations (detachment of device components), 70 involved perforation of the IVC, and 56 involved filter fracture.”28 The FDA raised concern that some of these events may be related to leaving retrievable filters in place for extended times, beyond the time when risk of PE has subsided. As a result, the FDA recommended that “implanting physicians and clinicians responsible for the ongoing care of patients with retrievable IVC filters consider removing the filter as soon as possible when protection from PE is no longer needed.”28 Once a retrievable filter has been placed, evidence has suggested that active protocols that monitor patients after filter placement and determine optimal timing of retrieval are more successful than passive protocols in terms of limiting loss of patients to follow-up and improving filter retrieval rates.29–32
In conclusion, retrievable filters should be considered in the following clinical scenarios: (1) indications for permanent filters are not present, (2) the risk of clinically significant PE is acceptably low, (3) return to high risk for venous thromboembolism is not anticipated, (4) life expectancy is long enough that the benefit of filter removal will be realized, and (5) the filter can be removed safely.7 Until further evidence is available, optional/retrievable filters should be used only when there is a temporary need to prevent PE in a patient with documented venous thromboembolism and when there is a defined retrieval end point, determined by when anticoagulation can be restarted safely and/or when PE risk has diminished, at which time optional filters should be actively retrieved. Optional/retrievable filters should not be used as replacements for permanent filters if permanent PE prevention is needed.
Specific Patient Groupings
Trauma Patients
The risk of development of venous thromboembolism in trauma patients is high, with a reported incidence of DVT without prophylaxis as high as 50% and incidence of PE as high as 30%.33,34 In a prospective study of 443 patients with major trauma not receiving any thromboprophylaxis, rates of overall DVT and proximal extension were 58% and 18%, respectively.35,36 Even with routine use of thromboprophylaxis, the respective rates of DVT and proximal involvement were 27% and 7%. Multiple independent risk factors for venous thromboembolism in trauma patients have been described, including advanced age, high Injury Severity Score, head injury, spinal cord or vertebral injury, pelvic and long-bone fractures, venous injury, and multiple transfusions. Prolonged immobility, venous stasis, and a proinflammatory hypercoagulable state contribute to this overall risk. The 2012 ACCP recommendation for trauma patients with at least one risk factor is to receive some form of thromboprophylaxis, preferably with an anticoagulant if there is no contraindication based on bleeding, or mechanical prophylaxis.37 For patients who cannot receive anticoagulation, the ACCP recommends mechanical thromboprophylaxis but does not support the use of vena cava filters for this purpose. Although venous thromboembolism prophylaxis with anticoagulation protocols is recommended by the 2012 ACCP and also by the 2002 Eastern Association for the Surgery of Trauma guidelines,38 there is a lack of level I evidence supporting any recommendation for or against filters.
Because of the risk of bleeding in severely injured trauma patients, standard pharmacologic thromboprophylaxis may not be possible. Routine prophylactic placement of filters has been recommended by some for use in trauma patients who were considered at very high risk for venous thromboembolism, with most series reporting a decreased incidence of all PE and fatal PE with the use of vena cava filters in appropriately selected high-risk patients.39–42 Level II and III evidence would indicate that the risk-to-benefit ratio of prophylactic filter placement is favorable in high-risk trauma patients. In a retrospective review of 9721 patients, high-risk categories included head injury plus spinal cord injury, head injury plus long-bone fracture, severe pelvic fracture plus long-bone fracture, and multiple long-bone fractures.43 A meta-analysis demonstrated a 0.2% incidence of PE in patients with filters, as opposed to 1.5% in controls without filters and 5.8% in historic controls.34 In a comparison of 40 critically injured patients who received filters and 80 matched historic controls, PE occurred in 3% versus 18%, respectively.44 At a 5-year follow-up of 132 trauma patients receiving prophylactic filters, Rogers et al45 reported a 2.3% incidence of PE after filter placement and a mortality of 4.4% with one fatal PE episode. Carlin et al42 compared a group of 122 trauma patients who received therapeutic vena cava filters for PE with 78 patients who received prophylactic filters and reported a mortality rate of 11% versus 3% and an incidence of recurrent PE of 18% versus 0%, respectively, with an associated 50% reduction in the overall incidence of PE over a 10-year period attributed to increased use of filters.
With the availability of optional filters there has been a growing trend in many trauma centers in the use of prophylaxis. Most studies evaluating retrievable filters in high-risk trauma patients have reported a high technical success rate and low incidence of PE, but the actual rate of retrieval has been variable.46 In a large multicenter study, 446 patients received a retrievable vena cava filter, with the majority (76%) being placed for prophylaxis, but only 22% were retrieved.47 Meier et al48 analyzed 35 trauma patients in whom prophylactic retrievable vena cava filters were placed; retrieval was possible in 86%, and 36% had trapped clot or thrombus within the device. In a study of 127 multitrauma patients with retrievable filters, Rosenthal et al49 reported that 52% underwent uneventful retrieval after pharmacologic prophylaxis was initiated. Of the remaining filters not removed, most were left because of contraindications to anticoagulation and only a few because of thrombus trapped within the filter. Although optional filters seem to represent an attractive option for high-risk trauma patients, there are unresolved issues, such as ensuring adequate follow-up and surveillance and the optimal timing of removal.
Analysis of the results of filter placement in trauma patients is limited mainly by the lack of adequate control groups, with most series using unmatched cohorts or historical data for comparison. Several reports and a meta-analysis of prospective studies found no difference in rates of PE in patients with and without prophylactic filters.50 To date, there have been no randomized clinical trials in which the prophylactic use of vena cava filters was shown to be more beneficial than other effective prophylactic measures. Until these issues are resolved by level I evidence, use of IVC filters is indicated in patients with proven DVT and a contraindication to therapeutic anticoagulation. On the basis of level II and III evidence in patients without documented DVT, use of vena cava filters as prophylaxis for thromboembolism may be considered only in those who have very-high-risk criteria and cannot receive anticoagulation.
Patients Undergoing Bariatric Surgery
Obesity is a strong independent risk factor for the development of venous thromboembolism. In a prospective study of more than 100,000 females, obesity was found to be one of the strongest independent risk factors for PE, with an adjusted relative risk of 2.9.51 PE is considered the most important cause of perioperative mortality in bariatric surgery patients, with an incidence of 1% to 3%, but the incidence has been reported to be as high as 17%, especially in superobese patients (body mass index >55 kg/m2).51–53 Combined with the added risk associated with surgery, the potential risk for venous thromboembolism is increased in this morbidly obese population.
Difficulty in proper dosing of anticoagulation thromboprophylaxis in obese patients increases susceptibility to venous thromboembolism–related morbidity and mortality. In a 2000 survey of American Society of Bariatric Surgery members, more than 95% of participants reported using prophylaxis routinely, and 38% used two prophylactic measures simultaneously.54 Despite the routine use of thromboprophylaxis, the incidence of PE in bariatric surgery patients has remained relatively stable, even with the addition of standardized heparin protocols and the application of minimally invasive approaches to bariatric surgery. Several investigators have compared various thromboprophylactic regimens, but no conclusive results on the safest and most effective form of anticoagulation have been reached.55–58 Furthermore, several studies suggest that the risk of PE after bariatric surgery extends to the period after hospital discharge, a finding more concerning with the shorter hospital stays in patients undergoing minimally invasive bariatric surgery.59,60
Because of these limitations of anticoagulation, placement of vena cava filters for thromboprophylaxis in patients undergoing bariatric surgery has become increasingly common. In a study of 5554 bariatric operations by Hamad and Bergqvist,61 risk factors for postoperative venous thromboembolism, including body mass index greater than 60, truncal obesity, and hypoventilation syndrome, were identified and recommended as indications for prophylactic filters. In a comparison of the incidence of venous thromboembolism in 248 high-risk patients undergoing bariatric surgery who received vena cava filters and 2852 low-risk patients who did not receive filters, Obeid et al62 showed that the incidence of PE was not significantly different. The use of prophylactic IVC filters reduced the risk for PE in high-risk obese patients, a group known to have a much greater incidence of morbidity and mortality, to a rate comparable to the baseline risk of a low-risk group. Several other studies using high-risk criteria for the use of prophylactic filters in patients undergoing bariatric surgery have demonstrated high technical success rates and a significantly decreased incidence of PE. In a series of patients undergoing open gastric bypass reported by Gargiulo et al,63 58 filters were placed prophylactically, with a 100% technical success rate and no postoperative PE. Similarly, Triquilio-Black et al64 placed filters safely with minimal morbidity and no PE in 41 high-risk bariatric surgery patients.
With the availability of optional/retrievable filters, there is the potential for using a vena cava filter as a “bridge” to keep the patient safe from PE during the highest-risk period and then removing it. Piano et al65 analyzed outcomes from a standardized approach in which retrievable filters were inserted in 59 patients undergoing bariatric surgery who met high-risk criteria. All filters were placed immediately before surgery, and removal was attempted 4 weeks postoperatively. There was one postoperative PE (1.7%) and no fatal PE or deaths. Halmi and Kolesnikov66 showed, in 27 high-risk bariatric surgery patients, that preoperative placement of retrievable IVC filters is a safe measure for the prophylaxis of PE and that most filters can be removed successfully within 2 to 3 weeks postoperatively.
However, evidence from these studies favoring prophylactic placement of vena cava filters in high-risk bariatric surgery patients is significantly limited by small study sample size, lack of prospective data, no adequate control populations, and variable approaches to venous thromboembolism prophylaxis. Given the relative infrequency of fatal PE after bariatric surgery, much larger trials are required before the indications for and efficacy of preoperative vena cava filter placement in the bariatric surgery population can be better defined.
Patients Undergoing Orthopedic Surgery
Patients undergoing major orthopedic surgical procedures, including total hip arthroplasty, total knee arthroplasty, and traumatic lower extremity fracture repair, have a significantly increased risk for venous thromboembolism. Several randomized studies have shown the incidence after major orthopedic surgery in patients not receiving prophylaxis to be 40% to 60% for any form of venous thromboembolism, 10% to 30% for proximal DVT, and 0.2% to 5% for fatal PE.37,67 With the routine use of perioperative thromboprophylaxis for major orthopedic surgery now accepted, the incidence of symptomatic venous thromboembolism has been reduced to 1.5% to 10% within 3 months, most events occurring after discharge, and fatal PE is now uncommon.68–72
The role of the IVC filter in elective major orthopedic procedures is not well delineated. Several studies support the efficacy of filter placement for the prevention of venous thromboembolism in selected orthopedic patients. In a cohort of 95 patients undergoing joint arthroplasty either with confirmed PE or at high risk for the development of PE in whom IVC filters were implanted, Austin et al73 showed that vena cava filter placement was effective in preventing fatal PE in all patients Golueke et al74 reported no filter-related complication or PE in 88 joint arthroplasty patients who received a vena cava filter in the perioperative period. Vaughn et al75 observed fatal PE in 1 of 24 patients undergoing total joint arthroplasty who also received filters and concluded that placement of an IVC filter is a safe, easy, and effective method of preventing fatal PE in selected patients undergoing joint arthroplasty.
Optional filters may be more appropriate in these high-risk orthopedic patients because they provide temporary protection at the time of increased risk and are intended to be retrieved when the risk of venous thromboembolism has diminished. Strauss et al76 reported on 58 high-risk orthopedic patients in whom retrievable IVC filters were placed as an adjunct to standard venous thromboprophylaxis. Minimal morbidity was associated with filter placement, and two deaths occurred unrelated to the filter or PE. Sixty-four percent of the filters were removed at a mean of 37.8 days, whereas others were left in place for up to 238 days, allowing them to serve as temporary prophylaxis and avoid the development of long-term complications. The researchers concluded that for high-risk orthopedic patients, placement of a retrievable IVC filter as an adjunct to standard pharmacologic or mechanical prophylaxis is an effective means of preventing PE. Retrievable filters have the advantage of acting as “bridges” in high-risk orthopedic patients during the period of most increased risk, but in the absence of randomized studies, their use should be limited at this time. One the basis of current evidence-based guidelines, either a permanent or an optional vena cava filter should be placed only in the high-risk orthopedic patient with documented venous thromboembolism and a contraindication to or complication of anticoagulation.
Patients with Cancer
Malignancy is an independent risk factor for the development of venous thromboembolism, with a reported incidence of 7% to 50% and a twofold to fourfold higher risk of PE in patients with malignancy than in those without it.77 Gitter et al78 found a 3.5-fold increased risk for venous thromboembolic complications in patients with cancer who were receiving anticoagulation therapy, and Prandoni et al79 found a 3.6-fold increase. Lin et al80 reported increased risk for recurrent venous thromboembolism after the development of new metastases or multiple episodes of neutropenia, especially in those with a history of venous thromboembolism. The risk of bleeding in patients with cancer while receiving anticoagulation is also higher. Bleeding complication rates in cancer patients treated with anticoagulation therapy have been reported to be as high as 35% to 50%.81 Gitter et al78 found a fourfold increase in bleeding in patients with cancer who were receiving anticoagulation therapy, and Calligaro et al82 reported a 25% incidence of bleeding in patients with advanced-stage malignancy.
Although some writers have suggested that primary filter placement in patients with malignant disease may be beneficial, others have favored anticoagulation.83–85 Lin et al80 suggested that vena cava filters may be a reasonable alternative to long-term anticoagulation in a subgroup of patients at high risk for recurrence, provided that the patients’ quality of life is reasonable and a decrease in the risk for fatal PE is justified. In a review of 308 patients, Wallace et al86 showed that filters can be placed in patients with venous thromboembolism and cancer without higher rates of procedure- or filter-related complications than in historical controls, although patients with a history of DVT and bleeding or metastatic/disseminated disease have the lowest survival.
The fiscal impact of filter placement in patients with cancer may be less than that of anticoagulation therapy because of the added costs of treating bleeding complications of anticoagulation therapy.87 Marcy et al88 reviewed 30 patients with malignancy in whom filters had been placed; 76% were alive at 1 month, 56% at 3 months, and 40% at 6 months. In a comparison of the complication rates and cost, vena cava filter placement was favored if reasonable survival was expected. Chau et al89 used a Markov model to compare the cost-effectiveness of filters versus anticoagulation. Vena cava filters were not cost effective in patients with brain tumors, but when the model was applied to the anticipated 5-year survival for a breast cancer population, vena cava filter placement appeared to be more cost effective than anticoagulation alone. From the available data, it appears that filter placement is most cost effective in patients with cancer and documented venous thromboembolism, good functional status, and anticipated survival.
Problematic with filters as primary therapy for patients with cancer is the finding of a higher number of venous thromboembolic events—PE, recurrent DVT, or vena cava thrombosis. The rate of recurrent venous thromboembolic events after filter placement in patients with cancer varies from 4% to 62%.90–92 Inhat et al,93 in a review of 166 cancer patients with venous thromboembolism, showed that although the rate of technical complications of filter placement is low, serious life-threatening or limb-threatening thromboembolic complications developed in 17% of patients, and survival was poor in all patients regardless of treatment, thus leading to support for a conservative approach consisting of routine anticoagulation therapy with selective filter placement. Because of poor survival in patients with advanced-stage malignancy, Jarrett et al,94 in a cohort of 116 patients undergoing vena cava filter placement, concluded that IVC filter placement may be of little clinical benefit and a poor utilization of resources. Because use of IVC filters as primary venous thromboembolism prophylaxis in patients with malignancy is not supported by evidence-based literature and because of the potential for further thrombotic events, use of such filters should be reserved for cancer patients with documented venous thromboembolism, contraindications to or complications of anticoagulation, and longer anticipated survival and should be discouraged in patients with advanced malignant disease and poor prognosis.
High-Risk General Surgery Patients
The incidence of DVT in general surgery patients not receiving prophylaxis varies between 15% and 30%, with rates of fatal PE between 0.2% and 0.9%.95–99 The type and duration of surgery clearly influence the risk for venous thromboembolism, with most outpatient procedures having low risk and more extensive inpatient procedures higher risks. Additional factors that raise the risk for venous thromboembolism in general surgery patients include the presence of standard risk factors such as malignancy, previous venous thromboembolism, obesity, varicose veins, and estrogen use; advanced age; type of anesthesia, with a lower risk with spinal/epidural anesthesia and a higher risk with general anesthesia; and perioperative factors, including the extent of mobilization, fluid status, and transfusion practices. On the basis of the results of numerous randomized clinical trials and meta-analyses, the ACCP 2012 consensus statement recommended the routine use of thromboprophylaxis after major general surgical procedures.37 Although mechanical methods of prophylaxis are attractive options in general surgery patients who are at high risk for bleeding, they have not been studied as extensively as pharmacologic thromboprophylaxis but do appear to reduce rates of DVT. The ACCP 2012 consensus statement recommends that mechanical prophylaxis be considered for patients who have a particularly high risk of bleeding.
Shorter hospitalization time leading to shorter duration of prophylaxis may heighten the risk for venous thromboembolism. Although the risk of postoperative DVT development is highest within 1 to 2 weeks after major general surgery, venous thromboembolic complications and fatal PE can occur later. In 51 patients who underwent major abdominal surgery, who received thromboprophylaxis in the hospital, and in whom DVT was excluded before discharge, evidence of DVT developed in 25% during the next 4 weeks.100 The optimal time for venous thromboembolism prophylaxis after major general surgery remains unclear, although newer guidelines suggest extending anticoagulation thromboprophylaxis for up to 28 days in high-risk patients.37 Prophylactic use of vena cava filters may be an attractive option to extend protection beyond hospitalization, but at this point there is no evidence to support this practice.
Overall, prophylactic use of vena cava filters for high-risk general surgery patients is not well defined. There are some perceived advantages of IVC filters in high-risk surgical patients in avoiding the bleeding risk associated with thromboprophylaxis and extending venous thromboembolism prophylaxis beyond discharge. In a registry of 1375 consecutive patients with DVT who had undergone recent major surgery, venous thromboembolism prophylaxis was used in only 44%, and IVC filters were more common in these surgical patients than in medical patients.101 Although filters are being used in high-risk surgical patients, there is no evidence supporting their use in this setting.
Use of vena cava filters in general surgery patients should be limited to the standard indications, that is, documented venous thromboembolism in patients with contraindications to or complications of anticoagulation. Although filters may be regarded as prophylaxis for high-risk patients who cannot receive anticoagulation therapy, current recommendations from the ACCP 2012 statement support only mechanical prophylaxis, not filters, in this population.37
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree