Vein Grafts for the Superior Vena Cava
John R. Doty
Donald B. Doty
Obstruction of the superior vena cava (SVC) can present either as a chronic, progressive syndrome or as an acute, life-threatening process. According to Nieto and one of the authors (D.B.D.)29 more than 90% of all cases of SVC syndrome (SVCS) are caused by malignancy, with lung carcinoma and lymphoma being the most common tumors. Lung cancer in particular has a propensity to cause obstruction of the SVC, with reports by Nogiere and associates30 and Escalante12 that 5% to 15% of patients with bronchial carcinoma develop SVCS. Benign causes are less common, with fibrosing mediastinitis and fungal diseases the leading etiologies, as noted by Kalweit and colleagues17 as well as by Nieto and one of the authors (D.B.D.).29 However, Abner1 has reported an increasing number of infectious causes of SVCS in the setting of immunosuppression. As observed by Escalante,12 thrombosis of the SVC results in the most dramatic presentation and is becoming more common with the increasing use of central venous access catheters and invasive monitoring devices.
The clinical features of SVC obstruction are discussed in Chapter 177 and are not repeated here. However, the diagnostic tests that should be used to guide medical and surgical therapy may be reiterated for emphasis; these include chest radiography, ultrasonography, computed tomography (CT), and contrast venography. According to Stanford and one of us (D.B.D.),39 venography is particularly important to properly stratify patients for surgical intervention. Patients with the type III (near complete to complete obstruction of the SVC with reversal of azygos blood flow) pattern of obstruction on venography are the best candidates for caval bypass operations.
Most cases of SVCS are treated effectively with nonoperative therapy, as noted in Chapter 177, especially if the etiology is infectious or malignant. Thrombolytic therapy, as noted by Gray and associates,15 has been shown to be useful in resolving SVC obstruction secondary to clot formation and is most effective in lysis of thrombus associated with a central venous catheter. Percutaneous placement of intravascular stents, as reported by Jackson and Brooks16 and Shah and coworkers,36 also has a role in SVC obstruction secondary to malignancy, but such stents are prone to thrombosis. Wisselink and colleagues43 have noted that simple balloon angioplasty yields results inferior to those of operative reconstruction and requires multiple attempts to approach the long-term success of surgery.
Indications for Surgery
The operative indications for surgical treatment of SVC obstruction are not completely defined and depend to some degree on the underlying etiology and the rapidity of the obstructive process. Malignant diseases that result in acute, sudden obstruction and thrombosis of the SVC may not resolve with thrombolytic and radiation therapy. Acute obstruction of the SVC associated with signs of cerebral or laryngeal edema are indicative of death within 6 weeks. Surgical intervention in these patients not only relieves life-threatening symptoms immediately but also allows definite treatment of the malignancy to proceed with the patient feeling more comfortable. Invasion of the SVC by malignant or benign tumor may be resistant to chemotherapy or radiation therapy because of clot and fibrosis associated with clot resolution, stimulated and propagated by the presence of tumor within the bloodstream. Obstruction of the SVC with restriction of blood flow may persist even though there appears to be good response of the tumor to treatment. Some of the benign causes of SVC obstruction are severe and relentless fibrotic processes that result in recurrent and extending obstruction. Clot propagation within the venous system proximal to the primary site of caval obstruction may present the appearance of progression of SVCS in patients with a benign etiology. Some patients with obstruction of the SVC may never develop adequate collateral circulation even though the causative process may be stabilized or arrested. Thus, any patient being considered for surgical reconstruction should have contrast venography documentation of complete obstruction of the SVC with inadequate collateral circulation.
Current recommendations for operative intervention in SVC obstruction are as follows: (a) persistent severe SVCS because of chronic SVC obstruction caused by a benign process, (b) acute SVC obstruction caused by a benign or malignant process with signs of cerebral or laryngeal edema, (c) relief of life-threatening SVCS during palliation of a malignant process, and (d) failure of nonoperative therapy to resolve SVCS caused by SVC obstruction.
Surgical reconstruction is contraindicated in patients in whom collateral circulation has formed adequately to provide upper compartment venous decompression. Also, patients with large, bulky tumors of the anterior mediastinum are unsuitable
candidates for operative intervention, as are those with limited life expectancy because of advanced malignancy or associated medical disorders.
candidates for operative intervention, as are those with limited life expectancy because of advanced malignancy or associated medical disorders.
Vein Graft Options
Operative reconstruction of venous drainage patterns in the thorax ideally should be performed with autogenous tissue to provide the optimal long-term outcome. Options for autologous venous conduits include spiral saphenous vein, femoral vein, straight saphenous vein, and composite autogenous vein grafts. In unusual settings and according to individual patient anatomy, alternative venous conduits—such as azygos vein–inferior vena cava or jugular vein–femoral vein grafts—can be constructed. In the absence of autogenous venous tissue, aortic, pulmonary, and venous homografts, bovine jugular vein, or pericardial tube construction can serve as conduit. Prosthetic graft materials are generally inferior to autogenous tissue grafts.
Success with bypass grafting of the SVC depends primarily on two main factors: adequate size of the conduit and proper orientation. The ideal graft should closely match the native SVC in diameter to prevent residual obstructive flow gradients. The graft should be measured carefully for length so that the completed conduit does not have redundancy, which can result in graft kinking and obstruction. Both the inflow and outflow of the graft should be free of intraluminal obstructions, such as atrial trabeculations and venous thrombosis.
Spiral Saphenous Vein Graft
The most extensive experience with venous bypass grafts has been with the spiral saphenous vein graft, a concept developed by Chiu and associates in 1974.7 The first use of the spiral vein graft in humans occurred in a patient with SVC obstruction secondary to granulomatous mediastinitis and was reported in 1976 by one of the authors (D.B.D.) and Baker.10 Twenty-three years later, this patient remained asymptomatic.
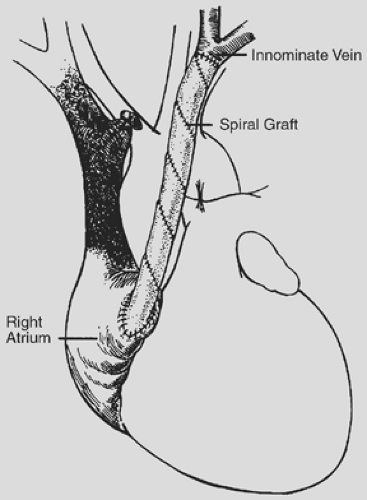
The spiral vein graft is constructed from the patient’s own saphenous vein and used as a bypass graft from the innominate vein to the right atrium (Fig. 178-1). After a sternotomy is performed, the distance from the confluence of the left internal jugular vein and the left subclavian vein to the right atrial appendage is measured. The diameter of the innominate vein is measured as the eventual diameter of the spiral vein graft. The saphenous vein is then exposed and its diameter measured. The length of the saphenous vein to be removed is calculated using this simple formula:
For example, if the innominate vein is 15 mm in diameter, the saphenous vein is 3 mm in diameter, and the length to the right atrial appendage is 8 cm, the proper length of saphenous vein to be harvested is 40 cm (15/3 × 8).
The saphenous vein is removed and incised in a longitudinal fashion throughout its entire length. A thoracostomy tube is chosen that is the same diameter as the innominate vein as a stent to form the bypass graft. The saphenous vein is flattened and wrapped in spiral fashion around the stent; the edges are then joined using a continuous suture of 7-0 polypropylene. This forms a large conduit with a diameter that closely matches that of the innominate vein and a length that approximates the distance from the jugular–subclavian confluence to the right atrial appendage.
Heparin (200–300 U/kg) is administered intravenously, and the innominate vein is ligated at its junction with the SVC. A soft jaw vascular clamp is applied at the jugular–subclavian confluence and the innominate vein is divided. Abnormal intimal tissue and thrombus are removed from the innominate vein. The spiral vein graft is pushed slightly off the stent, and an end-to-end anastomosis is performed to the innominate vein using continuous 7-0 polypropylene suture. The stent is then removed from the graft, and a curved vascular clamp is placed on the right atrial appendage. The tip of the right atrial appendage is excised, and obstructing trabeculae are removed to ensure unrestricted blood flow out of the graft. The graft is anastomosed to the right atrial appendage using continuous 5-0 polypropylene suture.
Most patients undergoing spiral vein bypass grafting for SVC obstruction can be followed clinically for graft patency. Prompt resolution of symptoms indicates successful decompression, and stenosis or occlusion of the graft is typically heralded by return of the obstructive syndrome. Ultrasonography, CT scanning, and venography, as well as magnetic resonance imaging, as noted by
Levitt and associates,22 are useful adjuncts for evaluating spiral vein bypass grafts when the postoperative clinical presentation is less clear.
Levitt and associates,22 are useful adjuncts for evaluating spiral vein bypass grafts when the postoperative clinical presentation is less clear.
Femoral Vein Graft
The first successful bypass operations for SVC obstruction were performed with autologous femoral vein grafts, as reported in the early 1950s by Bricker and McAfee6 and Klassen and associates.18 Since that time, however, the femoral vein has rarely been used as a bypass graft for SVCS. More recently, Gladstone and associates13 used autologous femoral vein to construct a bypass graft in two patients, one with mediastinal fibrosis and the other with poorly differentiated carcinoma. Lau and associates20 also described use of the autologous superficial femoral vein in two patients.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree