Esters
Amides
Procaine (Novocain®)
Lidocaine (Xylocaine®)
Chloroprocaine (Nesacaine®)
Mepivacaine (Polocaine® or Carbocaine®)
Bupivacaine (Marcaine®)
Prilocaine (Citanest®)
Ropivacaine (Naropin®)
Bupivacaine was first synthesized in 1957 and was of particular interest because of its long duration of action. Major side effects of bupivacaine include central nervous system and cardiovascular toxicity [3–6]. Ropivacaine is an alternative local anesthetic with fewer toxicities and is derived from the optically active isomers of mepivacaine [3, 7].
26.2.2 Pharmacology
The mechanism of action for local anesthetics is an alteration of sodium conduction across the neuronal cell membrane. The resting membrane potential is established across a neuronal membrane by the sodium/potassium ATPase pump. This results in a cell membrane with a negative electrical potential of about −70 mV. When a stimulus is applied to a neuron, there is an initial opening of the sodium channels and a positive change in membrane potential. When a certain threshold is met (approximately −55 mV), a larger opening of voltage-gated sodium channels produces an action potential which is propagated as an impulse along the neuronal cell (Fig. 26.1). This impulse also conducts pain signals from a peripheral nerve to the spinal cord and subsequently to the brain. Local anesthetics exert their effect mainly by blocking the sodium conduction necessary for the initiation and propagation of the action potential. Sodium channels are membrane proteins that consist of a large alpha subunit and one or two smaller beta subunits. The alpha subunit allows the passage of sodium ions [8, 9]. Local anesthetics bind to a specific site on the alpha subunit from inside the cell. The voltage-gated sodium channels exist in three states: the resting, activated, and inactivated states. Local anesthetics have a greater affinity for the sodium channel when in the inactivated and activated states as compared to the resting state; therefore, local anesthetics exert their greatest effect on nerves that are firing rapidly [10].
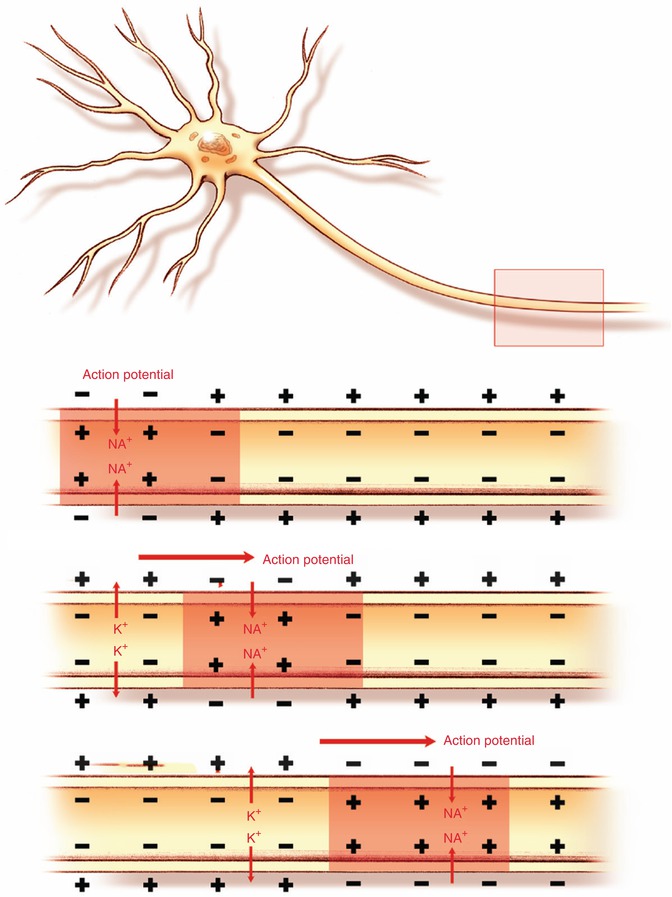

Fig. 26.1
Propagation of an action potential
Local anesthetics are compounds that exist in solution. The pKa of a compound in solution is the pH at which 50 % of the compound exists in ionic form and 50 % exists in nonionic form. The tendency to release hydrogen ion determines a compound’s strength as an acid, and the tendency to bind hydrogen ion determines its strength as a base. As the pH decreases, there are more hydrogen ions in solution and therefore a greater tendency for the compound to hold on to hydrogen. As the pH increases, there are fewer hydrogen ions in solution, and therefore it increases the tendency of the compound to release hydrogen into solution. Local anesthetics are weak bases. Structurally they exist as amino esters or amino amides. The amino group when bound to a hydrogen ion forms a charged species. It should be noted also that the amino group on local anesthetics has a pKa that is higher than physiological pH.
Therefore, if a local anesthetic has a low pKa or one that is close to physiological pH, it has a higher proportion of non-ionized species and can gain access to a neuronal cell better compared to a compound that has a high pKa; this is the case as non-ionized compounds tend to be more lipophilic and thereby penetrate the cell membrane more readily. An alternative explanation for the function of local anesthetics involves altering the fluidity of the neuronal cell membrane in such a way that the conformation of the sodium channel changes, thereby changing its conductance [2, 11]. When a local anesthetic has a pKa close to physiological pH, it has a higher concentration of its non-ionized, lipophilic form that can pass through the neuronal cell membrane; this translates into a faster onset [7]. Table 26.2 shows the physical properties (including pKa values) for the more commonly used local anesthetics [10–13].
Table 26.2
Physical properties of the commonly used local anesthetics
Concentration (%) | pKa | pH | Onset | |
|---|---|---|---|---|
Esters | ||||
Procaine | 0.25–0.5 | 8.9 | 3.5–5 | Fast |
Chloroprocaine | 1–2 | 9 | 4.5 | Fast |
Amides | ||||
Lidocaine | 1–2 | 7.7 | 5.0–7.0 | Fast |
Prilocaine | 1 | 7.7 | 4.5 | Fast |
Mepivacaine | 1 | 7.6 | 4.5–6.8 | Fast |
Bupivacaine | 0.25 | 8.1 | 4–6.5 | Fast |
Ropivacainea | 0.5 | 8.2 | 5.5–6.0 | Fast |
Local anesthetics in the form of esters are eliminated via plasma esterases, while the amides are absorbed into the circulation and eventually metabolized by the liver and excreted by the kidneys [1, 14]. When choosing which anesthetic to use for a field block, it is important to consider onset and duration of action and the suitability of tissue for a block. In addition, the use of a vasoconstrictor and the maximum dose of local anesthetic are of equal importance.
26.2.3 Onset and Duration of Action
Lipid solubility can affect onset of action for a local anesthetic (Table 26.2). Increased concentration of the drug (even if it is known to be a drug of slow onset) can speed up the initiation of the block; such is the case with chloroprocaine (pKa 9 and ionized proportion of 97 %). The onset and duration of action are also affected by the target tissue: highly vascular tissues may take the drug away from the site and limit its maximum effect. Uptake is slower for highly lipid-soluble drugs and those that avidly bind to protein. Generally, amides tend to have a longer duration of action than esters (Table 26.3).
Table 26.3
Maximum dosages allowable for the administration of infiltrative local anesthesia
Esters | Maximum dose (plain) (mg/kg) | Duration of action (plain) | Maximum dose (with epinephrine) | Duration of action (with epinephrine) |
|---|---|---|---|---|
Procaine | 5 | 20–30 min | 7 mg/kg | 30 min |
Chloroprocaine | 11 | 15–30 min | 14 mg/kg | 30 min |
Lidocaine | 4 | 30 min to 2 h
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|