Chapter 158
Vascular Trauma
Abdominal
Demetrios Demetriades, Kenji Inaba
Abdominal vascular injuries are the most common cause of death after penetrating abdominal trauma. The surgical exposure, control, and definitive management of these injuries may challenge the skills and judgment of even the most experienced surgeons. These can be made even more complex by any associated intra-abdominal injuries. Rapid transportation to a trauma center, early recognition of the injuries, early surgical intervention, excellent knowledge of the anatomy, and good surgical judgment are critical for optimizing patient survival.
Surgical Anatomy
For vascular trauma purposes, the abdomen is conventionally divided into three anatomic areas (Fig. 158-1):
• Zone 2 (left and right), which includes the kidneys, paracolic gutter, and renal vessels.
• Zone 3, which includes the pelvic retroperitoneum and contains the iliac vessels.
Some authors include a fourth zone in the perihepatic area that contains the hepatic artery, portal vein, retrohepatic IVC, and hepatic veins.
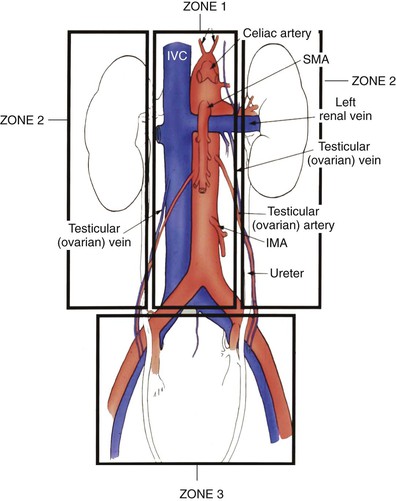
Figure 158-1 Retroperitoneal vascular zones. Zone 1 includes the midline vessels from the aortic hiatus to the sacral promontory; zone 2 includes the paracolic gutter and the kidneys; and zone 3 includes the pelvic retroperitoneum. IMA, Inferior mesenteric artery; IVC, inferior vena cava; SMA, superior mesenteric artery.
Epidemiology
Penetrating trauma is responsible for most abdominal vascular injuries and accounts for about 90% of cases in urban trauma centers.1 Low-velocity missiles cause direct injury to the vessel. High-velocity missiles and blasts can also cause vascular trauma by means of the shock wave and transient cavitation. Some of these injuries may be manifested as early or late thrombosis or hemorrhage.
In patients who undergo exploratory laparotomy for injury, the incidence of vascular trauma is 14.3% for gunshot injuries,2 10% for stab wounds,3 and 3% for blunt injuries.4
Blunt abdominal trauma may cause vascular injuries by one of three mechanisms:
Abdominal arterial and venous injuries occur with the same incidence. In a review of 302 abdominal vascular injuries from our center, the incidence of arterial injuries was 49% and that of venous injuries was 51%.1 The most commonly injured abdominal vessel was the IVC (accounting for 25% of injuries), followed by the aorta (21%), iliac arteries (20%), iliac veins (17%), SMV (11%), and SMA (10%). Overall, patients with penetrating trauma who sustained a vascular injury had an average of 1.7 vascular injuries per patient.1
Clinical Presentation
Many patients with major abdominal vascular injuries die at the scene and never reach hospital care. Of the patients who are transported to hospitals, about 14% lose vital signs during transportation or in the emergency department.1 The clinical presentation depends on the injured vessel, the size and type of the injury, the presence of associated injuries, and the time elapsed since the injury.
Penetrating injuries to the abdomen associated with hypotension and abdominal distention are highly suggestive of vascular injury. Asymmetrical femoral pulses may indicate iliac artery injury. Many patients may be normotensive on admission only to decompensate a few minutes later. Some patients present in a hemodynamically stable condition because of thrombosis of the vessel or effective containment of the bleeding in the retroperitoneum. In most cases, the diagnosis is made intraoperatively. Vascular injuries due to blunt trauma are often missed on initial examination or even during the initial hospitalization unless they are associated with significant bleeding or early ischemic changes.
Diagnostic Evaluation
In most patients with penetrating abdominal vascular injuries, no investigations are needed because of the patient’s critical condition and the obvious need for immediate laparotomy. Radiographic evaluation of the torso should be reserved only for gunshot wounds if the patient is hemodynamically stable. The location of any bullets or missile fragments on plain radiographs may be useful in planning the operation (Fig. 158-2) or in diagnosing other extra-abdominal injuries. About 30% of victims with gunshot injuries to the abdomen have multiple gunshot wounds,2 and an abdominal radiograph may provide useful information. Finally, early radiographic diagnosis of gunshot wounds to the spine or other bones may be useful in prescribing antibiotic prophylaxis. In blunt trauma, radiographic diagnosis of complex pelvic fractures may increase the suspicion of iliac vascular injuries.
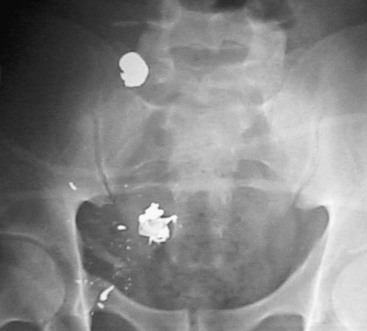
Figure 158-2 The pelvic location of the missile on the abdominal radiograph, combined with hypotension, is highly suggestive of an iliac vascular injury.
Although computed tomography (CT) has little or no role in suspected vascular injuries resulting from penetrating trauma, it may play a useful role in blunt trauma by identifying large hematomas, false aneurysms, or vessel occlusions (Fig. 158-3). In selected cases with penetrating trauma, elective CT after the intravenous administration of a contrast agent may identify false aneurysms or arteriovenous fistulae (Fig. 158-4). Angiography has no role in suspected vascular injuries resulting from penetrating trauma during the acute stage. However, it has an important role in the evaluation of suspected late complications, such as false aneurysms or arteriovenous fistulae. It remains a valuable tool that is both diagnostic and potentially therapeutic in the evaluation of patients with blunt trauma, especially those with pelvic fractures who are thought to have vascular injuries (Fig. 158-5).
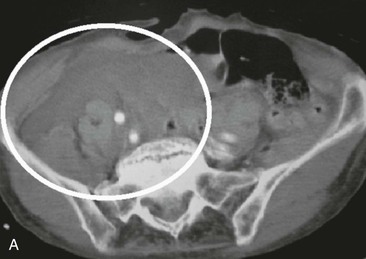
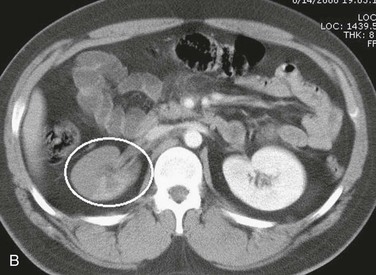
Figure 158-3 A, CT scan of a traffic accident victim shows a large pelvic hematoma (circle) due to injury of the right common iliac artery. B, CT scan with intravenous administration of contrast material in a high-speed traffic accident patient shows poor uptake of the contrast agent in the right kidney because of occlusion of the renal artery (circle).
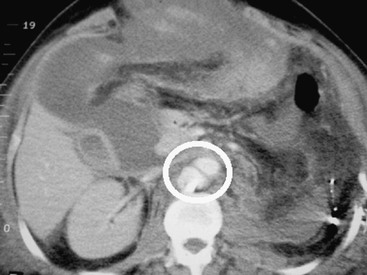
Figure 158-4 Postoperative CT scan of a patient with a gunshot wound shows an abdominal aortic false aneurysm (circle).
Treatment
Prehospital Treatment
The most important factor for the survival of salvageable patients with vascular injuries is rapid transportation to a trauma center followed by immediate surgical control of the bleeding. Prehospital advanced life support has no place in penetrating trauma, especially in an urban environment. A policy of “scoop and run” is currently the recommended approach. The role of prehospital intravenous fluid administration is controversial; some studies show improved survival with fluid restriction and others show no effect on survival.5,6 Experimental work on abdominal aortic injuries has shown that in the presence of uncontrolled bleeding, aggressive fluid resuscitation increases mortality and the rate and volume of hemorrhage.7,8 However, avoidance of all fluid resuscitation in near-fatal hemorrhage may result in cardiac arrest before bleeding is controlled.9 It seems that some degree of controlled hypotension is beneficial and prevents massive exsanguination while avoiding the risk of cardiac arrest due to massive blood loss and severe hypotension.7,10 Experimental work suggests that a systolic pressure of 80 to 90 mm Hg is the optimal pressure in the presence of active vascular bleeding.11 In many trauma systems, the prehospital intravenous resuscitation protocol recommends the insertion of intravenous lines and crystalloid resuscitation in the ambulance on the way to the hospital.
Emergency Department Treatment
The presence of hypotension or tachycardia (>120 beats/min) or a gunshot wound to the torso is a criterion for trauma team activation in most trauma systems. The type and duration of evaluation and resuscitation in the emergency department depend on the clinical condition of the patient. Endotracheal intubation and a resuscitative thoracotomy should be performed in the emergency department when patients are admitted in cardiac arrest or when cardiac arrest is imminent. A left anterolateral thoracotomy through the fourth to fifth intercostal space is performed, the thoracic aorta is cross-clamped, and the heart is massaged as necessary. If cardiac activity returns, the operation is completed in the operating room. The survival rate after emergency department resuscitative thoracotomy for abdominal vascular injuries is about 2%.1 We occasionally combine the emergency department thoracotomy with a laparotomy to control bleeding by direct compression, but the results are poor.
An attractive alternative to resuscitative thoracotomy is endoluminal aortic occlusion with use of a catheter balloon inserted through the femoral artery.12 The catheter may be inserted percutaneously, with or without ultrasound guidance. The balloon is placed and inflated in the distal thoracic aorta. The aortic occlusion reduces intra-abdominal bleeding and improves the resuscitation physiology. Experimental work comparing endoluminal balloon aortic occlusion to thoracotomy with aortic clamping in a model of hemorrhagic shock showed that aortic balloon occlusion was associated with increased central perfusion pressures with less physiologic disturbance.13 The available clinical experience with balloon aortic occlusion comes mainly from the management of ruptured aortic aneurysms.14 The experience with this technique in trauma resuscitation is limited, with sporadic case reports. Miura et al.15 reported good results in 7 of 10 patients undergoing laparotomy for hemostasis of massive hemorrhage.
In patients with suspected vascular injuries and in no need of resuscitative thoracotomy, time should not be wasted on fluid resuscitation or diagnostic investigations, with the exception of plain radiographs. Large-bore intravenous catheters should be placed in the upper extremities or the central veins of the thoracic inlet. Femoral vein catheters should be avoided in case the victim has an injury to the IVC or the iliac veins. The concept of controlled hypotension should be borne in mind, and aggressive fluid resuscitation should not be attempted in the emergency department. Except for patients in cardiac arrest or at risk for imminent cardiac arrest, endotracheal intubation should be avoided in the emergency department because rapid sequence induction is often associated with cardiovascular decompensation.
Surgical Treatment
General Principles
All possible steps should be taken to diminish hypothermia and its detrimental effects. The operating room should be warm, the infused fluids should be prewarmed to 40° C to 42° C, and the patient’s extremities should be covered with a warming blanket. Rapid infusion devices should be ready, and the blood bank should be notified. Once a vascular injury is identified, these patients should be resuscitated with blood products. Aggressive replacement of shed blood with products in a 1 : 1 : 1 ratio directed by a massive transfusion protocol has been demonstrated to improve outcomes. The patient’s entire torso, from the neck to the knees, should be prepared and draped in case thoracotomy or saphenous vein harvesting is necessary. The surgical team should be ready, and the skin preparation should be performed before induction of anesthesia because it is often associated with rapid hemodynamic decompensation in these patients.
Some surgeons have advocated a preliminary left thoracotomy and aortic cross-clamping to prevent cardiovascular collapse after anesthesia and laparotomy.16,17 The effectiveness of this procedure has been challenged by other authors.18 We believe that this approach should be considered only for patients in cardiac arrest or at risk for imminent cardiac arrest. A thoracotomy is an additional traumatic insult that may aggravate hypothermia and coagulopathy and has little effect on the control of bleeding from major venous injuries. We advocate an immediate laparotomy, temporary control of bleeding by direct compression, and aortic cross-clamping, if necessary, at the diaphragm. In our experience, this is almost always possible, even in obese patients. To facilitate aortic exposure, division of the left crus of the diaphragm may be necessary. In cases in which a retroperitoneal hematoma extends high toward the aortic hiatus, infradiaphragmatic exposure of the aorta is difficult, and a left thoracotomy may be necessary for aortic control.
Endoluminal aortic occlusion, as described before, is promising but still underused. The use of this technique can reduce the need for thoracotomy for proximal vascular control, eliminating the additional trauma and source of bleeding and hypothermia. In addition, it may make the exploration of anatomically difficult, upper zone 1 hematomas safer and easier. Training of surgeons in the use of this technique may add a useful tool in the armamentarium in the management of these complex cases.
The abdomen should be entered through a long midline incision. The operative findings depend on the nature and site of the vascular injury and the presence of other associated injuries. In penetrating trauma, the usual findings include various degrees of intraperitoneal bleeding, a retroperitoneal hematoma, or a combination of the two. In blunt trauma, the most likely finding is a retroperitoneal hematoma, which may or may not be expanding or pulsatile. In some patients with intimal tear and thrombosis, the hematoma may be unremarkable, and the only findings may be poorly perfused bowel or an absent or diminished femoral pulse. Some injuries may be missed only to be manifested at a later stage with thrombosis, false aneurysm, or arteriovenous fistula.
Retroperitoneal Hematoma
The management of retroperitoneal hematomas depends on the mechanism of injury. As a general rule, almost all hematomas due to penetrating trauma should be explored, irrespective of size. Underneath a small hematoma, there is often a vascular or hollow viscus perforation. The only exception to this recommendation is a stable and nonexpanding retrohepatic hematoma. Surgical exploration of the retrohepatic vena cava or the hepatic veins is difficult and potentially dangerous.
Retroperitoneal hematomas due to blunt trauma rarely require exploration because of the very low incidence of underlying vascular or hollow viscus injuries requiring surgical repair. In patients with zone 2 hematomas due to renal trauma, surgical exploration may result in the unnecessary loss of the kidney. Similarly, exploration of a zone 3 hematoma due to pelvic fractures may cause severe bleeding that may be uncontrollable. Exploration of retroperitoneal hematomas should be limited to patients with expanding, pulsatile, or leaking hematomas. In addition, zone 3 pelvic hematomas associated with an absent ipsilateral femoral pulse should be explored because of the potential for an iliac artery injury. Paraduodenal hematomas also need exploration to exclude an underlying duodenal injury. Finally, hematomas at the root of the mesentery in the presence of ischemic bowel may harbor an injury to the SMA and should be explored. Exploration of these hematomas is technically difficult and potentially dangerous and should not be performed in the absence of ischemic bowel. Unexplored hematomas should be evaluated postoperatively by means of color-flow Doppler studies (which may be difficult because of bowel gas), CT angiography, or angiography. With the advancement of endovascular technology and the availability of hybrid operating rooms with angiointervention capabilities, there is now the option to perform on-table diagnostic angiography instead of opening a contained retroperitoneal hematoma. Any significant injuries may be managed endovascularly, if amenable, with stenting or embolization. This approach has the additional advantages of avoidance of aortic cross-clamping and not opening the retroperitoneum, especially in the presence of associated hollow viscus injuries.
In the presence of severe active bleeding, the immediate priority is to control the bleeding by direct compression. Once this critical task is achieved, the next step is to identify the bleeding vessel and obtain proximal and distal control. If control is difficult or the patient is severely hypotensive, the abdominal aorta can be compressed digitally or with an aortic compressor at the aortic hiatus. After dissection of the peritoneum over the aorta and, if necessary, division of the left crus of the diaphragm (at the 2-o’clock position to avoid bleeding), the aorta can be cross-clamped. Endoluminal aortic occlusion with a percutaneously placed catheter balloon, as described before, is an attractive but still underused alternative.
The exploration of the area of bleeding or hematoma should proceed systematically. Each anatomic zone requires a different technical maneuver. Zone 1 supramesocolic bleeding or hematoma is the most difficult to approach because of the dense concentration of major vessels (aorta, celiac artery, SMA, renal vessels, IVC), the difficult exposure of many of these vessels, and the difficult proximal control of the infradiaphragmatic aorta. For some injuries, the only safe way to achieve proximal aortic control is through a left thoracotomy or with endoluminal aortic occlusion. The supramesocolic aorta, along with the origins of its major branches, is best exposed by mobilization and medial rotation of the viscera in the left upper abdomen. The first step of this approach is to divide the peritoneal reflection lateral to the left colon, the splenic flexure of the colon, and the spleen. The fundus of the stomach, spleen, tail of the pancreas, colon, and left kidney are then rotated to the right. This maneuver provides exposure of the aorta, origin of the celiac axis, SMA, and left renal vessels (Fig. 158-6). Some surgeons prefer not to include the left kidney in the medial rotation.19 However, for injuries involving the posterior wall of the aorta, inclusion of the left kidney in the visceral rotation improves the exposure. In suspected supramesocolic IVC injuries, zone 1 should be explored through a medial rotation of the right colon and hepatic flexure and Kocher mobilization of the duodenum and head of the pancreas (Fig. 158-7). The inframesocolic zone 1 area can be approached by retracting the transverse colon cephalad and displacing the small bowel to the right. The peritoneum over the aorta and IVC is then incised, and the vessels are exposed. An alternative approach is medial rotation of the right or left colon.
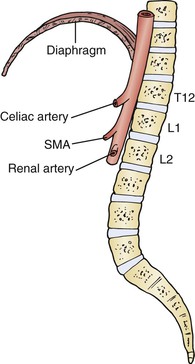
Figure 158-6 Medial left visceral rotation provides good exposure of the supramesocolic aorta and the origin of the celiac axis, superior mesenteric artery (SMA), and left renal vessels.
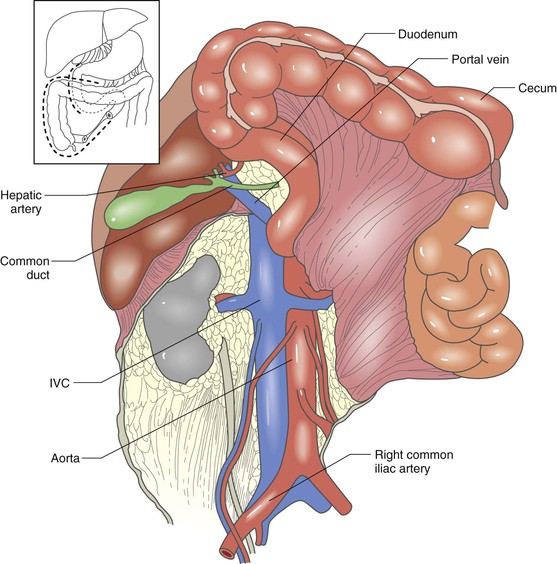
Figure 158-7 Medial rotation of the right colon and hepatic flexure and Kocher mobilization of the duodenum and pancreas provide excellent exposure of the inferior vena cava (IVC) and the origins of the renal veins. (From Buckman RF, et al: Injuries of the inferior vena cava. Surg Clin North Am 81:1431-1447, 2001.)
Zone 2 bleeding or hematoma is explored by mobilization and medial rotation of the right colon, duodenum, and head of the pancreas on the right side or the left colon on the left side. The source of bleeding in zone 2 is the renal vessels or the kidneys.
Zone 3 vessels are explored by dissection of the paracolic peritoneum and medial rotation of the right or left colon. In some cases, direct dissection of the peritoneum over the vessels provides the necessary exposure.
The reconstruction of major vessels with synthetic grafts in the presence of intestinal spillage poses significant risks for graft infection. Copious irrigation before graft placement, use of autologous graft material whenever possible, and omental wrapping or soft tissue coverage for polytetrafluoroethylene (PTFE) grafts will reduce the risk of infection.
Damage Control Procedures
With the damage control approach, all complex venous injuries are ligated, arterial injuries may be shunted, and any diffuse retroperitoneal or parenchymal bleeding is controlled by tight gauze packing. If commercially produced vascular shunts are not available, temporary intraluminal shunts can be constructed from sterile intravenous or nasogastric tubing.20 The shunt is secured with proximal and distal ligatures (Fig. 158-8). The abdomen is then closed temporarily with a prosthetic material or vacuum dressing techniques (Fig. 158-9), and the patient is transferred to the intensive care unit (ICU) for further resuscitation. The abdomen should never be closed primarily because of the very high incidence of abdominal compartment syndrome. Some patients may benefit from early angiointervention before admission to the ICU. The patient is returned to the operating room after resuscitation and stabilization for definitive vascular repair and abdominal wall closure.
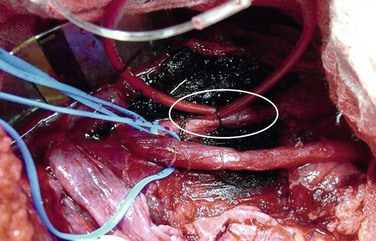
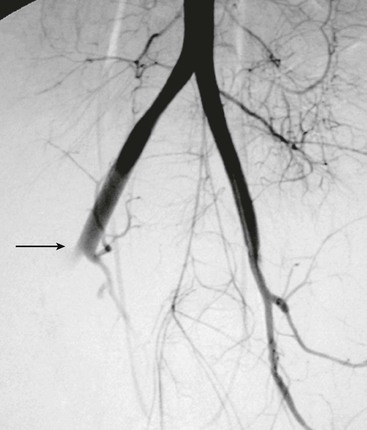
Figure 158-8 Temporary arterial shunt for damage control in a hemodynamically unstable patient with a gunshot wound and complete transection of the iliac artery (circle).
Endovascular Techniques
Endovascular techniques have revolutionized the management of blunt thoracic aorta and other arterial injuries. Hybrid operating rooms (Fig. 158-10) with angiographic radiology capabilities provide an opportunity to improve the management of abdominal vascular injuries by either temporary control of life-threatening bleeding or definitive care with angioembolization or stenting. In the appropriate cases, the combination of endovascular adjuncts and open surgery may be lifesaving.
The role of endovascular techniques in penetrating abdominal vascular trauma, which is almost always associated with severe active bleeding, is usually limited. However, the use of endoluminal balloons for temporary proximal aortic control can be a useful adjunct during open surgery. In addition, endovascular techniques may be the best therapeutic intervention in most cases with delayed vascular complications such as aneurysms, arteriovenous fistulae, and arterial occlusions.
Abdominal Compartment Syndrome
The normal intra-abdominal pressure in the resting supine position is near zero. Elevation of the intra-abdominal pressure above 12 mm Hg constitutes a diagnosis of intra-abdominal hypertension.21 A pressure that exceeds 20 mm Hg in addition to organ dysfunction results in abdominal compartment syndrome. Abdominal compartment syndrome is characterized by a tense abdomen, tachycardia with or without hypotension, respiratory dysfunction with high peak inspiratory and plateau pressures in mechanically ventilated patients, and oliguria. However, significant organ dysfunction may begin long before classic abdominal compartment syndrome is manifested.
All patients with severe abdominal trauma, especially vascular trauma, are at risk for development of abdominal compartment syndrome. Major risk factors include massive blood transfusions, prolonged hypotension, hypothermia, aortic cross-clamping, damage control procedures, and tight closure of the abdominal wall. After severe trauma, the abdomen should never be closed under tension. Similarly, after damage control procedures, the abdominal wall fascia should not be closed because progressive postoperative bowel edema will frequently result in abdominal compartment syndrome in most patients.
The diagnosis of abdominal compartment syndrome is based on clinical examination and intra-abdominal pressure measurements. All high-risk patients and those in whom abdominal compartment syndrome is suspected clinically should be monitored closely with serial intra-abdominal pressure measurements. The intra-abdominal pressure can be measured reliably through the bladder catheter. The catheter is used to empty the bladder. Approximately 20 mL of sterile saline is injected back into the bladder to allow pressure transduction without the false elevation in pressure expected from bladder wall tension in a full bladder. The pressure is then measured through the catheter by either a simple water column or a commercial pressure transduction device. Definitive management requires surgical decompression. In general, pressures higher than 20 to 30 mm Hg are considered strong indicators for surgical decompression of the abdomen. If organ dysfunction is present, the pressure threshold may be lower. The abdomen can be opened in the operating room or even in the ICU if necessary. Temporary abdominal wall closure can be achieved with the use of a commercially available suction device, or a sterile x-ray cassette cover, laparotomy sponges, closed suction drains, and sticky drapes can be used to accomplish temporary coverage (see Fig. 158-9). When the bowel edema improves, usually within 2 to 3 days, the patient is returned to the operating room for definitive abdominal wall closure. The technical details of temporary or permanent wall closure are beyond the scope of this chapter.
Specific Vascular Injuries
Abdominal Aorta Injuries
Anatomy
The aorta descends into the retroperitoneum between the two crura of the diaphragm at the T12-L1 level and bifurcates into the common iliac arteries at the L4-L5 level, which roughly corresponds to the level of the umbilicus. The first branches of the abdominal aorta are the phrenic arteries that originate from its anterolateral surface. Immediately below is the celiac trunk that originates from the anterior surface of the aorta, and 1 to 2 cm below is the SMA; the renal arteries are next, at 1 to 1.5 cm below the origin of the SMA; and finally, the inferior mesenteric artery (IMA) is 2 to 5 cm above the bifurcation of the aorta.
Mechanism of Injury
Blunt injury to the abdominal aorta is extremely rare, diagnosed in 0.04% of all blunt trauma admissions.22 Fractures of the thoracolumbar spine and seat belt injuries are associated with an increased risk of abdominal aortic injuries.22,23 Intimal dissection and thrombosis are the most common lesions in patients reaching the hospital alive. False aneurysms occur less frequently. Patients with free ruptures die at the scene and rarely receive medical care.23,24 In a review of 28 cases with blunt abdominal aortic injuries, intimal tear or large intimal flap was the most common lesion (60%), followed by free rupture (30%) and pseudoaneurysm (10%).25
Penetrating injuries are by far the most common cause of abdominal aortic injuries. In a review of 1218 patients with abdominal gunshot injuries from our center, there were 33 abdominal aortic injuries (2.7%). In 529 knife wounds to the abdomen, the aorta was injured in 8 (1.5%).26 The infrarenal aorta is injured in 50% of patients, the supraceliac aorta in 25%, and the aorta between the celiac trunk and the renal arteries in 25% of patients.27
Clinical Presentation
The clinical presentation depends on the mechanism of injury (blunt or penetrating), type of aortic injury, presence of free intraperitoneal bleeding or retroperitoneal hematoma, associated injuries, and time elapsed since the injury. In blunt trauma, two thirds of patients have acute symptoms of bleeding or visceral or lower extremity ischemia. The diagnosis is made during the initial hospitalization by means of CT or angiography or at laparotomy. In one third of cases, the diagnosis is made many months or even years after the injury.22–24
The clinical presentation of penetrating aortic injuries is usually dramatic. Many victims die at the scene and never receive medical care. Of those who are treated, about 28% have an unrecordable blood pressure, and about 21% require an emergency resuscitative thoracotomy.26 In about 18% of cases, the bleeding is temporarily contained in the retroperitoneum, and the patients are normotensive on admission.27 On rare occasions, the injury is missed at operation only to be manifested at a later stage as a false aneurysm or arteriovenous fistula (see Fig. 158-4).
Management
Almost all patients with penetrating aortic injuries require open repair. In blunt trauma, the management depends on the location and type of the injury. Many patients with small intimal tears can safely be managed nonoperatively. More severe blunt injuries with free rupture or large intimal flaps require open or endovascular repair.25 In a National Trauma Data Bank analysis of 436 patients with blunt abdominal aortic injuries, 394 patients (90%) were managed nonoperatively and 29 (7%) underwent endovascular repair, with only 13 patients (3%) undergoing open aortic repair or extra-anatomic bypass.28
Endovascular Treatment.
Endovascular management has a definitive role in selected cases of infrarenal aortic injury. Patients with limited infrarenal aortic dissection, large intimal flaps, false aneurysms, or aortocaval fistulae have been treated successfully with angiographically placed stents.29,30
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree