Chapter 159
Vascular Trauma
Extremity
David S. Kauvar, Larry W. Kraiss
Based on a chapter in the seventh edition by Kaushal R. Patel and Vincent L. Rowe
This chapter covers the epidemiology, diagnosis, and management of vascular injuries to the extremities. The focus is on arterial injuries distal to the deltopectoral groove (axillary artery and distal) and the inguinal ligament (common femoral artery and distal) resulting from blunt and penetrating noniatrogenic trauma. As the workup and management of such injuries have changed significantly during the past few years, the entire chapter has been rewritten, capturing recent data while retaining elements describing traditional (and in most cases still useful) management techniques.
Epidemiology and Pattern of Injury
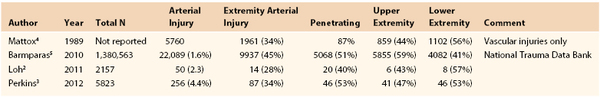
Trauma represents a leading cause of morbidity and mortality around the world.1 The overall incidence of trauma and vascular injuries is increasing, and although vascular injuries are present in only 1% to 2% of injured patients, these patients account for a far greater share of morbidity, mortality, and resource utilization than those without such injuries do.2,3 In modern series, vascular injuries to the extremities occur in 0.5% to 1% of injured patients but account for 20% to 50% of all vascular injuries (Table 159-1).2–5 As is typical for the general population of injured patients, those with extremity vascular injuries tend to be young, with average ages in the 30s, and predominantly (70%-90%) male.2–4
Mortality
Extremity vascular injuries result from blunt and penetrating mechanisms with nearly equal frequency in modern reports. Patients with blunt injuries experience mortality rates between 2% and 5%, whereas penetrating injuries generally result in far fewer deaths. The discrepancy is predominantly due to a much higher incidence of concomitant nonvascular injuries in patients sustaining blunt trauma. In a comprehensive, single-center study from the United Kingdom, Perkins et al3 reported double the rate of packed red blood cell use and massive transfusion as well as a hospital length of stay three times longer in patients with blunt injuries than in those with penetrating injuries, indicating a more severe overall injury complex. In some instances, however, mortality can be attributed to the extremity vascular injury itself, and in such cases penetrating injuries to proximal extremity arteries predominate. Dorlac et al6 identified 14 cases of prehospital exsanguination from isolated extremity vascular injuries. All of these injuries resulted from penetrating mechanisms, and 79% of patients exsanguinated from an artery proximal to the knee or elbow. Similar results were noted in an autopsy study by Bilgen et al,7 who found that a penetrating mechanism was responsible for 95% of lethal extremity vascular injuries and that 84% of these injuries were to proximal arteries. We performed a study of isolated lower extremity arterial injury revealing a 2.8% mortality rate and 78% of deaths due to penetrating trauma. Mortality increased as the level of arterial injury became more proximal.8
Amputation
Amputation rates in patients with extremity vascular injury range from 7% to 30% in modern series, with most amputations performed in patients with blunt mechanisms.3,8 The high amputation rates seen in patients with blunt vascular injuries to the extremities can be attributed to the high-energy mechanism of such injuries and the resultant amount of damage to bone, nerve, and soft tissue and not typically to the vascular injury itself.8–12 Amputation is most common with arterial injury at the forearm level in the upper extremity and at the popliteal level in the lower extremity.5,8,12 The influence of vascular disruption on the decision to amputate or to reconstruct limbs sustaining combined trauma to multiple tissue types is explored later in this chapter.
Location of Injury
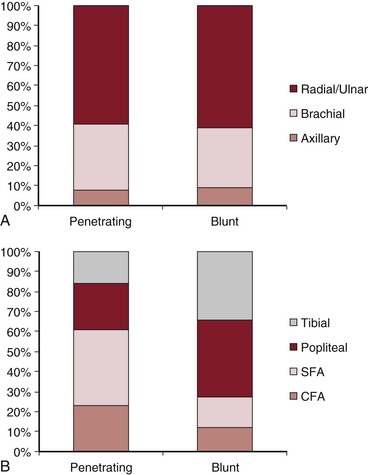
In large series examining both blunt and penetrating mechanisms, arterial injuries occur with nearly equal frequency in the upper and lower extremities.4,5,13 In the upper extremity, regardless of mechanism, the most frequently injured arterial level is that of the forearm vessels (radial and ulnar arteries). In the lower extremity, however, mechanism influences the arterial injury pattern, with the popliteal artery most frequently injured in blunt trauma and the superficial femoral artery (SFA) most frequently injured in penetrating trauma (Fig. 159-1). A blunt mechanism of injury is seen more frequently in lower extremity than in upper extremity arterial injuries. Lower extremity injuries have accordingly higher incidences of associated tissue injuries, complications, and mortality.13
Associated Tissue Injuries
In extremities sustaining arterial injuries, other tissues are injured with varying frequency based on the mechanism and arterial level of injury. In general, fractures are seen with high frequency in blunt limb trauma, with rates as high as 80% to 100% in some series.14,15 In penetrating trauma, fractures are seen in only 15% to 40% of limbs with an arterial injury.15–17 Regardless of mechanism, fractures, especially comminuted ones, consistently represent a risk factor for amputation when they are found in association with extremity vascular injury.8,14–16
The incidence of named venous injury concomitant with extremity arterial trauma ranges between 15% and 35% in most studies. The presence of a vein disruption does not consistently predict amputation, and there is no consensus as to whether such injuries should be repaired and whether failing to do so predisposes the patient to long-term morbidity. Long-term limb edema in particular appears to be unrelated to whether a venous injury was repaired or not.14
The presence of a peripheral nerve injury associated with extremity vascular trauma can be difficult to detect acutely, and thus such injuries may be underreported in most of the literature on extremity vascular trauma. For the lower extremity, most studies report rates around 10%; upper extremity vascular trauma is associated with a much higher incidence of nerve injury, around 40% to 50%. Like concomitant vein injuries, a nerve injury does not predict amputation, even in severely injured limbs.18
Significant soft tissue disruption often accompanies extremity vascular trauma. As with nerve injuries, the incidence appears to be higher in the upper extremity, reported as 40% to 70%, than in the lower extremity, for which rates are typically reported to be 30%. The presence of a significant soft tissue deficit does appear to correlate with amputation in lower extremity arterial trauma.14,15
Diagnosis and Workup
Although uncommon, vascular injuries to the extremity are associated with significant morbidity and mortality. Thus, the efficient diagnosis and rapid localization of such injuries are of great importance in the evaluation of a trauma patient. In cases in which a patient presents with “hard signs” of vascular (primarily arterial) injury, the existence and location of the injury are generally obvious, and the next step in management is clearly operative exploration and repair. The diagnosis of injuries in patients presenting with only “soft signs” of vascular injury is more challenging, and such patients typically require specific diagnostic modalities in addition to the secondary trauma survey (Box 159-1). Conventional catheter-based angiography, once considered the “gold standard” for the preoperative diagnosis and localization of extremity vascular injuries, has been largely supplanted by other diagnostic techniques and is now mostly reserved for intraoperative use in the modern management of these injuries.
Physical Examination and Doppler Indices
A number of studies speak to the diagnostic sensitivity of the combination of physical examination and measurement of Doppler-derived blood pressures in detecting extremity arterial injuries resulting from penetrating trauma. Frykberg et al19 studied 310 patients with penetrating injuries to the extremities, most of whom presented with proximity of the wound to a major vascular structure as the sole outward sign of vascular injury. Physical examination alone identified 21 of 23 major arterial injuries, and all patients with hard signs of injury on examination were found to have a significant injury; most patients with an injury presented with more than one hard sign. This resulted in a positive predictive value of physical examination alone of 100% and a negative predictive value of 99%. A prospective study in which routine angiography was used to screen for arterial injury in patients with soft signs was performed by Weaver et al.20 They found that proximity alone was a poor predictor of significant arterial injury, with no surgically significant arterial injuries identified in the population in whom proximity was the sole indication for angiography. They also found that an isolated Doppler-derived pressure index of 0.99 or less (arterial pressure distal to presumed injury/normal brachial arterial pressure) had a sensitivity of 82% and a specificity of 40% for the detection of a significant arterial injury. These results were confirmed in a similar prospective study by Schwartz et al,21 who found a sensitivity of 86% for a pressure index of less than 1.0. In both of these studies, a pulse deficit in the injured extremity correlated very well with the presence of a significant arterial injury. For upper extremity injuries and in situations in which both lower extremities are injured, the pressure used to calculate an “injured extremity index” may be obtained from any uninjured extremity with reliability similar to that of the ankle-brachial index.20,21
Fewer specific data are available on the reliability of physical examination and Doppler indices in detecting arterial injury in blunt extremity trauma, but the excellent results in penetrating injuries have been extrapolated to this population. Rozycki et al12 cited a 66% rate of pulse diminishment and a 31% rate of pulselessness in a population of bluntly injured extremities with arterial injuries. In a series of 500 blunt popliteal artery injuries, Wagner et al14 similarly reported a pulselessness rate of 94% and a diminished pulse rate of 4%.
All patients presenting with trauma to the extremities and hard signs of arterial injury should have those injuries surgically explored in an expeditious manner. Patients presenting with soft signs should undergo a complete pulse examination and a measurement of Doppler pressures in the injured limb distal to the suspected injury for the calculation of a pressure index. If the index is less than 1.0, a further diagnostic and localization study should be performed. The proximity of an extremity wound to a named vessel alone should not prompt a localization study in the absence of a diminished or absent pulse or a Doppler index of less than 1.0.
Computed Tomographic Angiography
Computed tomographic angiography (CTA) is the diagnostic modality most frequently used to diagnose and to localize extremity arterial injuries in modern trauma, having largely supplanted conventional catheter-based angiography (Figs. 159-2 and 159-3). Most seriously injured patients undergo contrasted CT scans as part of their initial evaluation, and scans are frequently completed within minutes of a patient’s presentation to a trauma center. CTA offers the benefit of imaging the bone and soft tissues of the injured extremity simultaneously with the arterial interrogation. In addition, CTA can assist in the diagnosis of major venous injuries if a delayed phase scan is performed.
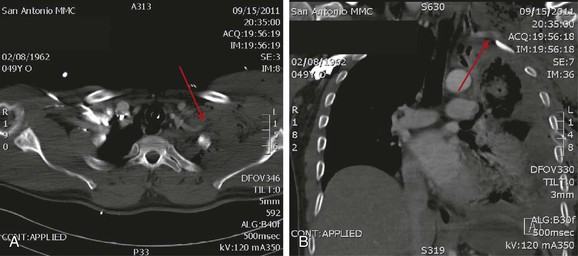
Figure 159-2 CTA images from a patient sustaining a gunshot wound to the left shoulder with a pulseless left upper extremity. Note abrupt truncation of the left subclavian artery as it enters a large hematoma (A, arrow). Coronal reconstruction images reveal occlusion with intraluminal filling defect at the thoracic outlet (B, arrow).
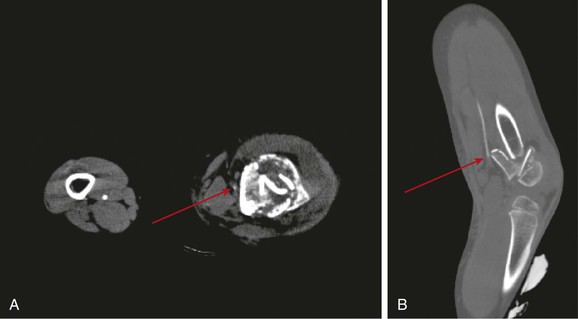
Figure 159-3 CTA images from a patient sustaining blunt trauma to the left lower extremity with comminuted femur fracture. Note diminutive (likely spasm) left superficial femoral artery at the level of the fracture (A, arrow) just proximal to its abrupt truncation, seen best on reconstructed images (B, arrow).
In a review of imaging in vascular trauma, Patterson et al22 compiled nine studies published from 2001 to 2009 covering 540 patients undergoing CTA to diagnose extremity arterial injuries. There were a total of seven missed injuries, for a rate of 1.3%. The sensitivities reported in the nine studies averaged greater than 95%, with specificities greater than 90%. Seamon et al23 performed a prospective study of CTA for 21 patients with extremity injuries in which all patients received CTA followed by conventional angiography. The criterion for entry was a Doppler index of less than 0.9 in the injured extremity. CTA identified nine injuries, all of which were confirmed at operation or by conventional angiography. No significant arterial injuries were found with conventional angiography after being missed on CTA, although three cases of arterial spasm were discovered in this manner and successfully treated nonoperatively. In this prospective study, CTA was obtained within minutes, whereas angiography took more than 2 hours on average. CTA was also associated with a cost savings of more than $15,000 per patient.
CTA does have some limitations in the evaluation of limb trauma. Many studies report inconsistent contrast opacification of the arterial system if the timing of the intravenous bolus is not optimal. In addition, imaging artifact from metal fragments can limit diagnostic utility. In patients with a large number of fragments, such as those with shotgun injuries, conventional angiography may offer better diagnostic capability than CTA. Despite these limitations, CTA should be considered the initial diagnostic and localization modality of choice in patients presenting with soft signs of extremity arterial injury, especially in those whose Doppler index is less than 1.0. Conventional angiography should be reserved for unique cases and intraoperative use.
Duplex Ultrasonography
The use of duplex ultrasonography (DUS) in the diagnosis of extremity vascular trauma has been limited, especially with the increasing use of CTA. DUS can be time-consuming and operator specific, but the existing data regarding the technique deserve mention here. Bynoe et al24 performed a prospective study in a mixed population of 198 blunt and penetrating extremity trauma patients. All patients had a wound in proximity to a major vessel and underwent DUS. In cases in which DUS was inconclusive or the trauma surgeon thought more information was needed, arteriography was performed. DUS identified 22 major arterial injuries, 12 to the SFA. Only two injuries were missed (one diagnosed on angiography and the other during a surgical wound débridement); both were due to shotgun wounds. The sensitivity and specificity of DUS for the diagnosis of arterial injury were 95% and 98%, respectively. Bergstein et al25 performed a similar study in which 67 penetrating extremity trauma patients with soft signs of arterial injury or proximity underwent arteriography followed by DUS. Four arterial injuries were identified on angiography, but only half of these were also seen on DUS, making the sensitivity only 50% compared with a gold standard of arteriography. Finally, Gagne et al26 published a small study in which only four arterial injuries were prospectively identified, three discovered only by arteriography and one only by DUS, confirming the questionable reliability of DUS in the trauma setting. In this study, however, DUS was also compared with contrast venography for the detection of venous injuries. DUS identified seven injuries, four of which were missed by venography, which captured only one injury (to a calf vein) missed by DUS.
Although DUS is inexpensive and noninvasive, it is unreliable for the detection of arterial injuries in extremity trauma. CTA remains the most appropriate initial study for this purpose. DUS may have a role in the detection of occult venous injuries and should be considered an adjunctive imaging modality in extremity trauma. In addition, DUS may have a role in the surveillance of known injuries selected for nonoperative management (see later).
Treatment Principles
Nonoperative Management
The presence of an arterial injury in a traumatized extremity does not mandate surgical therapy. With the decreasing application of routine arteriography in patients without clear outward signs of arterial injury, many clinically occult lesions may in fact go unidentified. The results presented in the studies cited in the diagnosis section speak to the insignificance of these injuries in terms of requiring operative repair. Stain et al27 reported on 45 extremity arterial injuries noted on routinely performed post-trauma angiography and treated initially without surgical exploration. Injuries included small, non–flow-limiting intimal defects and flaps, small pseudoaneurysms, and arteriovenous fistulae. None of the injuries produced distal ischemia, nor was there free extravasation. Of these 45 injuries, 31 (69%) were proximal to the knee or elbow. None of the 45 progressed to require operative intervention after a mean of more than 5 weeks of follow-up.
Dennis et al28 presented a dual series of extremity trauma patients including 44 patients with an arterial injury identified on angiography at presentation and undergoing initial nonoperative management. Requirements for initial nonoperative management included a lack of distal ischemia or extravasation and a defect smaller than 2 cm. Of the 44, 4 (9%) developed new hard signs of vascular injury (all within 1 month of injury) and went on to be repaired. All of these were new or enlarging pseudoaneurysms. Additionally reported in this series were 309 patients with injuries in proximity to a major vessel. These patients were evaluated without angiography and observed clinically. Four (1.3%) eventually developed hard signs of injury (all within 1 week of injury) and had operative repairs. No long-term limb morbidity resulted from their approach in either group of patients.
These results confirm the lack of necessity for routine arteriography and suggest that nonoperative management of selected arterial injuries is reasonable, but a high index of suspicion for the possible development of a pseudoaneurysm or ischemic complication is necessary during a period of close clinical follow-up. Injuries identified on imaging that may be considered for observation include those that produce no active hemorrhage or distal ischemia, such as small intimal tears or flaps, pseudoaneurysms, and arteriovenous fistulae. Serial surveillance with an appropriate imaging modality such as CTA or DUS is recommended.
Endovascular Therapy
The use of endovascular techniques to treat extremity vascular injuries has undergone significant growth as these techniques have become integrated into vascular surgical practice during the past 2 decades. From 2000 to 2003, there was a nearly fourfold increase in the percentage of arterial injuries treated by endovascular means. This same period saw a greater than 30-fold increase in the use of stent-grafts to treat arterial trauma.29 Endovascular therapy is currently applied more frequently in blunt than in penetrating trauma and in lower extremity than in upper extremity injuries. It appears to have similar or improved outcomes compared with open repair of extremity arterial injuries despite being applied in patients with a significant burden of associated injuries and medical comorbidities.30
Being less invasive than open surgery, endovascular treatment of extremity arterial injuries seems most appropriate when the morbidity difference between the open and endovascular procedures is greatest. This is most often the case in the setting of injuries to junctional vessels (such as the subclavian and iliac).31 In such cases, the use of endovascular balloon occlusion for proximal vascular control may obviate the need for opening a body cavity. If the traumatic vascular lesion can be safely traversed with a guide wire, a definitive endovascular treatment can be performed.
Stent-grafts have been successfully deployed to treat arterial injuries manifesting with hemorrhage and occlusion.32 The greatest experience is in the iliac and subclavian vessels, and reported short-term results are promising.33,34 Catheter-directed embolization with coils and glue has been used to treat smaller vessels and seems particularly successful when it is used for the treatment of small pseudoaneurysms and arteriovenous fistulae of the crural and deep femoral branch arteries.35,36
With endovascular equipment and skills in increasingly ready supply, their broader application in the treatment of extremity vascular injuries seems inevitable. Enthusiasm for their use should be tempered by the fact that there are few or no long-term data available on the outcomes when these techniques are used “off label” to treat such injuries. Every limb trauma patient is different, and the most appropriate treatment should be determined on a case-by-case basis through close communication between the trauma surgeon and endovascular provider. If an institution is to have the capability to apply endovascular techniques to emergently treat extremity trauma patients, it must be prepared with the ready availability at all hours of the equipment and expertise necessary to maximize the benefits of these techniques.
Open Surgical Management
Open surgical control and repair remain the mainstays of the management of most extremity vascular injuries. If possible, operations should be performed on a table amenable to fluoroscopic imaging of the injured extremity. This can facilitate orthopedic treatment of associated bone injuries and on-table angiography if needed. The patient should be prepared and draped widely to facilitate exposure, typically circumferentially in the case of extremity injuries. If the need for a vein graft is anticipated, the proposed harvest site should be included in the prepared operative field. Conventional practice is to obtain saphenous vein grafts from uninjured extremities to preserve collateral venous drainage. This dogma is not supported by good-quality data, however, and it may be appropriate to harvest conduit from an injured extremity if there is no venous injury present.
The general principles of establishing proximal and distal vascular control and restoring in-line flow to a suitable outflow bed are the same for the treatment of traumatic lesions as for any other vascular defect. Because traumatic vascular injuries frequently present with significant hemorrhage, the ability to achieve vascular control expeditiously is paramount. Pneumatic operative tourniquets, placed in a sterile or unsterile fashion, can be used to provide rapid control of inflow until formal arterial control proximal to a bleeding injury can be obtained. To ensure adequate exposure for control and repair, incisions are typically made longitudinally, directly over the target vessel proximal and distal to the injury. Once vascular control is achieved, the incisions can be extended as needed to expose the zone of vascular injury. In some cases of extremity injury, control may need to be obtained proximal to the extremity itself. Such cases lend themselves well to the endovascular placement of an occlusion balloon to achieve proximal control. If this is not possible, upper extremity control may require infraclavicular or supraclavicular incisions to expose the subclavian vessels. Retroperitoneal or inguinal ligament–splitting incisions may be required for proximal control in the lower extremity. Many trauma patients may be unable to tolerate systemic anticoagulation because of ongoing hemorrhage or associated injuries. In these patients, local anticoagulation with heparinized saline injection directly into the injured vessel proximal and distal to the injury may be used to prevent local thrombosis.
Wide débridement of contaminated and nonviable tissue within the zone of vascular injury should be performed. During débridement, an assessment of the availability of healthy tissue to cover the vascular repair should be made, as graft or repair coverage is essential to prevent desiccation and possible anastomotic breakdown and subsequent hemorrhage. Tissue coverage may require coordination with other services that may be operating on the injured extremity as well. The injured vessel should be débrided to healthy-appearing tissue before a formal plan for restoration of continuity is made. Intimal injury can extend beyond what is appreciated by an injured vessel’s outward appearance. Before heparinization and repair, the inflow and outflow vessels should undergo gentle balloon catheter thrombectomy to confirm patency. A subjective assessment should be made of the bleeding forward and backward from the injured vessel, and if it is not thought to be adequate, a search for additional flow-compromising injuries should be undertaken.
In general, provided the patient’s physiologic status will tolerate it, arterial injuries should undergo surgical repair with the goal of restoring distal flow. In some cases, however, acute ligation of an injured extremity artery may be the most feasible acute option. Sometimes this may be done with a plan for formal revascularization when the patient’s overall condition stabilizes. If an artery proximal to the elbow or knee is ligated, the extremity distal to the injury should be observed extremely closely for signs of developing ischemia, which should prompt immediate formal revascularization or shunting (or amputation if neither of these can be safely performed).37,38
Once the extent of the arterial injury is apparent, three options exist for repair. In some cases, a short injured segment can be resected circumferentially and enough of the vessel freed proximally and distally to perform a spatulated end-to-end anastomosis of the injured vessel. In other cases, a single arteriotomy can be débrided and a vein patch angioplasty performed. In many cases, however, neither of these “simple” options is feasible, and a vein interposition graft of appropriate length must be performed. All open traumatic wounds are considered contaminated, and the preferred conduit for repair of vascular injuries is autologous saphenous vein harvested from an uninjured extremity. Infections involving prosthetic grafts are notoriously difficult to manage, resulting in high rates of graft explantation and amputation. These issues are exacerbated when there is inadequate soft tissue coverage of the graft.39,40
It is generally accepted that the poor long-term patency seen with prosthetic grafts used for chronic occlusive disease will be manifested in trauma patients as well.41 Feliciano et al40 reported an early (≤30 days) graft occlusion rate of more than 6% in a series of 175 extremity arterial prosthetic grafts, with an additional 6% of grafts occluding after 30 days. The prosthetic arterial grafts most at risk for thrombosis were those placed in smaller vessels. Despite the perception of a high failure rate, the use of prosthetic conduits remains controversial, and no long-term patency data are available in cases in which such grafts are placed to repair vascular injuries. A short-segment prosthetic graft may be considered to repair uncontaminated (typically blunt) arterial injuries to large vessels, such as the axillary and common femoral arteries, where size match with the saphenous vein may be a problem.
Temporary Shunting
The civilian use of temporary shunts to expeditiously restore flow before definitive vascular repair in an injured extremity has grown out of initial military surgical experiences with the technique in the Vietnam War.42 Since that time, the technique has gained wide acceptance as a temporizing measure allowing distal perfusion during fracture stabilization before formal vascular repair or as an adjunct to damage control management of a patient unable to undergo definitive repair acutely (Fig. 159-4). In reports of shunts used to allow orthopedic fixation, arterial shunt dwell times have been in the range of 1 to 3 hours, with patency rates approaching 100% in the absence of the use of systemic anticoagulation.43–45 Dwell times are predictably longer when shunts are placed as part of damage control management, typically ranging from 12 hours to more than a day, allowing time for resuscitation and physiologic stabilization before definitive vascular repair. Despite higher limb injury severity in such damage control patients, shunt patency and limb salvage results are equivalent to those experienced when arteries are shunted to allow orthopedic stabilization.44 Although infrequently used, arterial shunts placed in the forearm and tibial vessels have poor patency (12% vs. 86% for proximal shunts) as presented in a recent military report.46 Shunts placed in the venous system fare surprisingly well in terms of patency despite the absence of systemic anticoagulation and dwell times of up to a few hours. In a combined review of two recent military reports, only a single episode of thrombosis was noted among 14 shunted vein injuries (93% patency).46,47
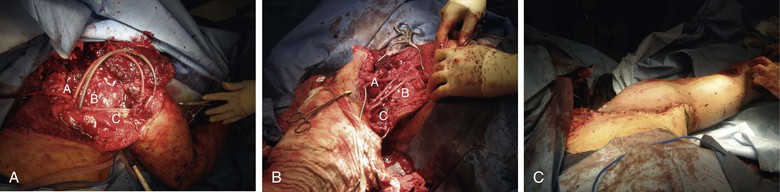
Figure 159-4 A, Open scapulothoracic dissociation (torso to the left) before return to normal anatomic length with axillary arterial (A) and venous (B) shunts in place. Note brachial plexus component (C) on significant stretch before reduction. B, After reduction, fixation, and interposition vein grafts to the axillary artery (A) and vein (B), the brachial plexus (C) is returned to its typical anatomic position and tension. C, Normal-appearing external shoulder anatomy is restored after skin closure.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree