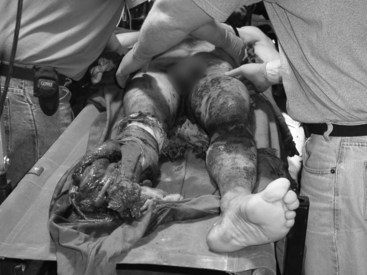
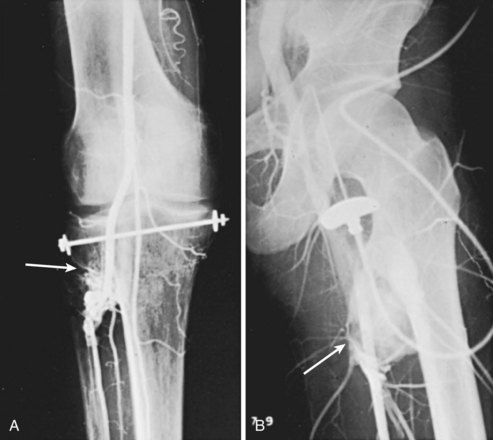
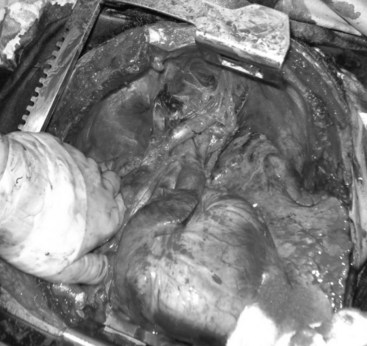
Chapter 45 Vascular Trauma
Background
The social effects of trauma are substantial; approximately 40 million emergency department visits and 2.6 million hospitalizations result annually from traumatic injuries.1 Patients younger than 45 years sustain 75% of the total lifetime cost of injury, an aggregate measure of the health care costs and societal costs over the patient’s lifetime from all injuries occurring in a given year. Societal costs include lost lifetime productivity, lost wages, and the need for social support such as unemployment wages and workers compensation. In both civilian and combat settings, the incidence of vascular injury is approximately 0.2% to 7% of all injured patients.2–8 Males represent the overwhelming majority of victims of vascular trauma specifically in nearly all anatomic locations,9–27 leading some to label vascular trauma as a disease of young males.28
It is important to consider both the similarities and differences in civilian and military vascular injury. While many surgical advances have taken place during wartime and we have learned a great deal from these experiences, the degree and pattern of injuries seen in civilian settings are generally different from those seen in military trauma. Among civilian victims, for example, gunshot wounds are responsible for the majority of peripheral vascular injury, stab wounds for a smaller percentage, and the remainder is due to blunt trauma.10,29 Because most civilian gunshot wounds are generally due to low-velocity projectiles, the degree of associated trauma is limited, usually involving only venous or neural injury. This is in contrast to military trauma, in which arterial injury usually occurs in the setting of massive extremity involvement, where orthopedic fractures, large soft tissue defects, and nerve and vein injuries are common. This pattern is seen in one third of patients presenting with vascular injury to combat trauma receiving centers; it can also be seen in civilian close-range shotgun blasts. The majority of military traumatic injuries are due to explosive devices (74%), with high-velocity gunshot wounds being responsible for most of the remainder (17%); blunt injury is infrequent.6,30
Early Control of Hemorrhage
The most common cause of potentially survivable death is uncontrolled hemorrhage, in both the military and civilian settings.31–35 Patients sustaining major vascular injury exhibit severe physiologic derangements resulting from hemorrhage, tissue hypoxia, and the sequelae of anaerobic metabolism (Figure 45-1). Severe hemorrhage leads to what has been termed the lethal triad of hypothermia, coagulopathy, and acidosis. Patients requiring massive transfusions, generally defined as requiring greater than 10 units of packed red blood cells, suffer mortality rates of 20% to 50%.36 Historically, trauma management algorithms have emphasized crystalloid fluid resuscitation and early administration of packed red blood cells to support cardiac output and tissue-level delivery of oxygen, with administration of plasma and platelets based on subsequent laboratory analysis. However, recent research has called this practice into question. In 2007, Borgman and colleagues37 reported that the ratio of blood products was related to casualty mortality at a combat support hospital. Subsequent research has documented similar findings in military38,39 and civilian40 populations.
The following principles constitute the damage control resuscitation approach to reversal of the lethal triad. The first step is identification of patients who require damage control management, as opposed to the physiologically stable patient who will tolerate definitive treatment of injuries in the initial operative setting. Once the decision is made to pursue a damage control strategy, an assessment of the patient’s immediately life-threatening issues must be performed. The basic tenet of damage control surgery is to stop ongoing contamination and hemorrhage and transport the patient as rapidly as possible to a resuscitative environment, such as the intensive care unit, in order to halt progression of the lethal triad. Any large vessel bleeding should be controlled surgically and repaired in an expeditious fashion. Any sources of gross contamination, such as injury to the gastrointestinal tract, should be controlled as well. If possible, vascular continuity should be restored by either an expeditious definitive repair or a temporizing measure, such as a vascular shunt. However, life must take precedence over limb, and if it is impossible to restore blood flow without exposing the patient to severe risk from a prolonged repair, ligation of the artery and management of the ischemic sequelae is preferable to losing the patient through a well-intentioned but overly heroic effort to save a limb. Acidosis should be prevented or treated. Hypothermia, found to be an independent predictor of mortality among combat casualties,41 must also be prevented or treated. Packed red blood cells are infused along with plasma and platelets in a 1 : 1 : 1 ratio, minimizing the use of red cells prior to vascular control and repair, and minimizing the use of crystalloid in general. Finally, new data support the early use of recombinant factor VII.42 Fibrinogen has been evaluated as part of this protocol and is a predictor of survival; however, a typical unit of fresh frozen plasma has 400 mg of fibrinogen, which supplies the recommended dose if at least a 1 : 2 ratio of packed red blood cells to plasma is maintained.39 Additional adjuncts such as tourniquets (which will be discussed in the Extremity Vascular Injury section) and hemostatic dressings such as Quickclot (zeolite powder; Z-Medica, Wallingford, Conn.) or HemCon (chitosan-based bandage; HemCon Medical Technologies, Portland, Ore.) are also used in combat environments to aid in rapid hemostasis. Tourniquet use has been shown to have such sufficient effects on outcome that it has become a part of most prehospital management algorithms in the civilian community.
To address concerns that damage control resuscitation might have a deleterious effect on vascular reconstructions, Fox and colleagues43 performed a case-control study of battlefield casualties who underwent vascular repair and compared patients treated with a rigorous damage control resuscitation protocol versus conventional crystalloid and nonmatched erythrocyte resuscitation. There was no worse outcome among patients when the coagulopathy was treated. The author’s admonition was to “fix the injury, fix it well, and trust that your patient doesn’t need to be coagulopathic for the graft to work.”43
In 1993, Rotondo and colleagues44 coined the term damage control surgery, referring to a limited surgical procedure or set of procedures with discrete, life-saving goals and the intent to defer more definitive repair until further resuscitation has occurred. The quandary of a patient in extremis requiring a further surgical insult to correct a life-threatening injury has been recognized for generations. Debakey and Simeone2 commented that while some injuries are clearly beyond any repair the patient could tolerate, others are complicated by the physiologic cost to the patient of that repair. Hughes stated in his report on arterial injuries in Korea that “the need for adequate amounts of blood and complete resuscitation in such individuals is stressed … rushing the improperly resuscitated patient to surgery in an attempt to save a limb may possibly result in loss of life.”5 The spectrum of procedures and maneuvers classified as damage control techniques has been growing as experience with this concept broadens, but the basic principles include rapid surgical control of bleeding, control of sources of contamination, and deferral of definitive procedures until the patient is more stable. Damage control options for vascular trauma include tourniquet use, temporary vascular shunting (TVS), ligation, or balloon tamponade.45–53 These specific techniques will be discussed in later sections. At this point, it is difficult to associate any particular damage control surgery technique with improved outcomes, because the numbers are too small and the confounding variables in patients in extremis are too numerous to achieve statistical power. Taken as a whole, however, damage control for major trauma is associated with improved physiology, increased limb salvage, and decreased mortality.40,43,45,46
Diagnosis of Vascular Injury
Manual palpation of pulses is the next step. Carotid, subclavian (in the infraclavicular recess), brachial, radial, ulnar, femoral, popliteal, and pedal pulses should be palpated and documented. The use of continuous wave Doppler ultrasound to augment the physical examination is appropriate. In 1971, Lavenson and colleagues54 evaluated the auscultation of Doppler signals in the distal pulses as a means of extremity pulse assessment, as an extension of the physical examination.54 The benefit of this technique is that it is both rapid and, if a sphygmomanometer is used in concert, can be quantified as the ankle-brachial index (ABI). The disadvantage, however, is that it is an indirect, blind study and does not exclude vascular injury. ABI has been found to be highly sensitive for clinically significant vascular injury. Patients with ABI greater than 0.9 may be observed, and angiography is reserved for those with ABI less than 0.9.55
Arteriorraphy has been the gold standard for diagnosis of vascular injury and remains an indispensable tool for the management of vascular trauma. It has a number of indications, including evaluation of the need for surgery, investigation of a suspected injury, and operative planning. It is indicated for blunt trauma with associated fractures, penetrating injury to the chest, zone I and III cervical injury, multiple pellet wounds, injuries to the forearm or leg, and knee dislocation. In the recent decades, there has been a great deal of effort to identify patients at risk for occult vascular lesions (Figure 45-2). In a series of 538 patients, Conrad and colleagues56 documented no missed injuries in the setting of a normal physical examination and normal ABI was associated with no missed injuries and concluded that angiography is unnecessary in this population. The limitation of this study was that there was only 51% follow-up of those discharged without further workup.56 In a series of 507 asymptomatic patients, however, Reid and colleagues57 documented abnormal arteriograms in 6.7%. In another study, 157 patients with penetrating extremity injuries but no hard signs of vascular injury underwent arteriorraphy; 11% of patients had abnormal arteriograms, of which 27% were major abnormalities.58 Gillespie and colleagues reported 90 patients with penetrating extremity injuries without hard signs and found 27% to have abnormal arteriograms; all injuries were successfully managed nonoperatively.59 A critical distinction must be made from a diagnostic standpoint between combat-related and civilian trauma. In combat settings, there is a higher rate of occult injuries found with arteriorraphy than in the civilian literature (45% versus 11%), most of which are associated with normal physical examination, and a higher percentage of these findings require operative or endovascular intervention (10% versus 1%).60
With the advent of high-resolution multislice computed tomography scanners, the ability to perform noninvasive arteriorraphy is changing the diagnostic algorithm for vascular injury. A series of 20 patients with combat-related vascular injuries were examined using a 64-slice multidetector computed tomographic angiography (MDCTA). Injuries were identified in 10 of 20 patients (50%) and the study was deemed adequate in 94% of cases. Four patients underwent comparative studies and there were no discordant findings. MDCTA seems to yield sufficiently high resolution images to be useful for the early evaluation of combat casualties. The presence of metallic fragments or orthopedic hardware did not interfere significantly in this report with the value of MDCTA.61
Finally, duplex ultrasonography should be considered in general for the diagnosis of vascular injury. Although sensitivity of this modality is variable and it is arguably operator dependent, some vascular territories are highly amenable to duplex examination (e.g., cervical vascular trauma). Its main advantages are relative speed of the study, noninvasive nature, and repeatability in order to follow injuries.62
Thoracic Vascular Injury
Vascular injuries involving thoracic vessels are some of the most lethal injuries that patients can sustain. These patients can be divided into three groups. The first group dies immediately at the scene of trauma from rapid exsanguination. The second group becomes unstable en route to medical care, and the majority (>96%) die secondary to multisystem trauma. If patients are able to survive with thoracic vascular injuries to medical care, their chance of survival goes up substantially to approximately 70% to 95%, with deaths in this group often being secondary to neurologic injury.63 These injuries require a significant investment of hospital resources. A recent study identified that patients treated at a level I trauma center had lower mortality from major thoracic vascular injury than at a level II center.64
There are a variety of findings suggestive of thoracic great vessel injury. These include hypotension, upper extremity hypertension, intermittent paralysis or focal neurologic findings, inequality of blood pressure or pulses between extremities, external evidence of chest trauma, an expanding hematoma at the thoracic outlet, an intrascapular murmur, a palpable fracture of the sternum or the thoracic spine, or a left-sided flail chest.63 The initial management of these patients should follow the standard trauma resuscitative pathways. Fluid resuscitation should be minimized for several reasons. If there is a full-thickness vascular injury, minimization of fluid resuscitation and permissive hypotension will help to stabilize early soft-platelet plugs. Any patients with military antishock trousers in place and the suspicion of a proximal vascular injury should have them removed immediately, because this is effectively a distal cross-clamp and can lead to instability of clots and worsened bleeding.63 If there is blunt injury, aggressive prevention of hypotension helps to avoid propagation of the injury. Finally, concomitant pulmonary contusions or other injuries are common, and minimization of fluid resuscitation will avoid iatrogenic worsening of respiratory status.
In cases of penetrating injury, patients with thoracic vascular injury generally are hemodynamically unstable. Patients with blunt thoracic vascular injury are generally hemodynamically stable, because those who are unstable from this mechanism die in the prehospital setting in almost 100% of cases. This distinction is critical, because a patient with blunt trauma who arrives in stable condition and subsequently turns unstable is most likely bleeding outside of the thoracic cavity.63
Penetrating Injury
The most common mechanism of injury to thoracic great vessels is penetrating trauma, responsible for 90% of injuries. In a series of 3823 gunshot wounds or stab wounds to the chest or abdomen, the aorta was injured in 1.6%. Most of these injuries were due to gunshot wounds (69%), with stab wounds (18%) and shotgun wounds (12%) composing the remainder of penetrating thoracic injuries. Whereas thoracic aortic injury was less common than abdominal aortic injury (28% vs. 72%), it was associated with a decreased likelihood of having a recordable blood pressure (27% vs. 71%), increased need for emergency department thoracotomy (EDT, 77% vs. 21%), and decreased survival (8% vs. 24%). Most patients with penetrating injury to the thoracic aorta arrived in shock (83%), with half of these patients demonstrating no recordable blood pressure. Mortality was 81% overall in this cohort, with gunshot wounds having a higher associated mortality (88%) than stab wounds (65%). Predictors of mortality in this series were gunshot wounds, injury to the thoracic aorta as opposed to the abdominal aorta, a nonrecordable blood pressure on admission, and the need for an EDT. Although concomitant injury to other structures occurred in 81% of patients, most commonly the lung, there was no association with mortality.4
Plain film radiography in these patients may reveal findings suggestive of great vessel injury, such as mediastinal widening, a foreign body out of focus with other structures indicating possible intracardiac location, a foreign body in close proximity to vascular structures, a confusing missile trajectory suggesting possible missile embolism, or a missile that is altogether missing, which suggests possible distal embolism. Radiopaque markers should be placed at the entrance and exit wounds to delineate missile trajectories.63
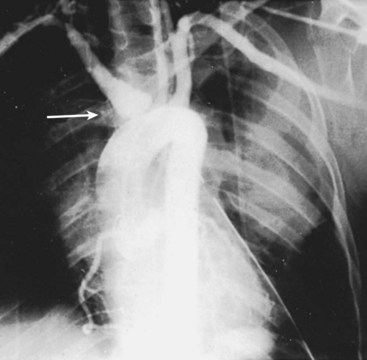
Anatomically, the aortic arch is oriented in an anterior posterior direction, and the left subclavian artery originates off of the aorta in the posterior mediastinum. As a result, it is nearly impossible to obtain rapid proximal control of this artery through a median sternotomy. In a patient with a penetrating injury above or below the left clavicle, the best approach to control the left subclavian artery is through an incision in the third interspace of the left chest. This high anterolateral thoracotomy will allow adequate visualization of the root of the left subclavian artery to gain vascular control. Once bleeding is controlled, the surgeon can explore the injured subclavian artery through standard supraclavicular incision (Figure 45-3).
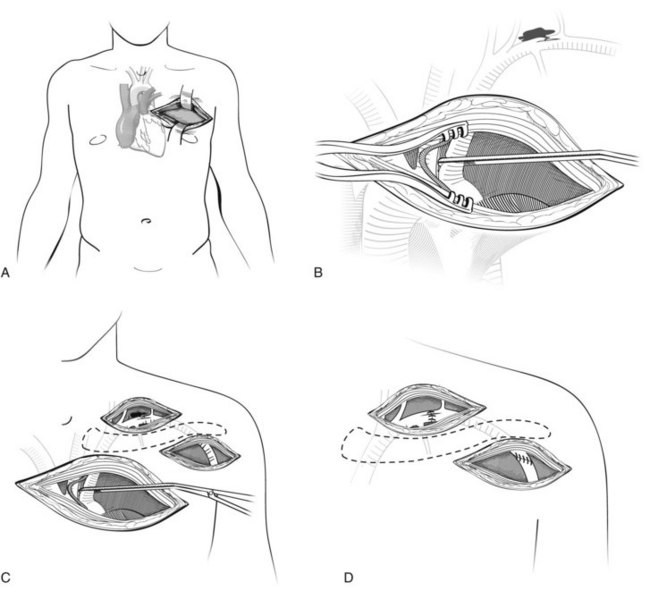
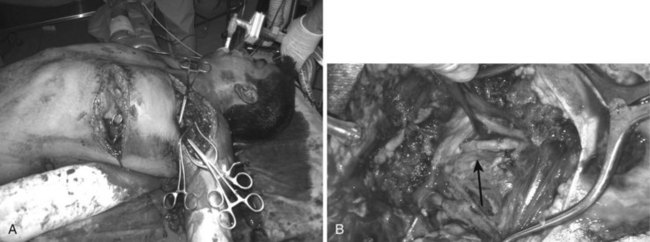
The most versatile exposure for penetrating thoracic trauma is a left anterolateral thoracotomy through the fourth interspace. It is not an accident that this is the same incision used for the EDT. The left side of the mediastinum including the pericardium and the descending aorta can be accessed through this incision. If this does not provide sufficient access, the incision is extended across the midline via a transverse sternotomy and can be continued into a right anterolateral thoracotomy in the third interspace. This is known as the clamshell incision (Figure 45-4). It is a highly morbid procedure, but if it is necessary, the patient is near death and all maneuvers are considered heroic at this point.
In the patient with a known site of injury, alternative incisions may be used. One of the most common approaches to the thoracic cavity is the midline sternotomy. An incision is made vertically from the sternal notch to the xiphoid process down to the sternum. Using blunt dissection, a retrosternal space is established, and a sternal saw is used to divide the sternum longitudinally. The Finochietto retractor is then placed and the thoracic cavity is opened. This affords access to the ascending aorta, the brachiocephalic vein, and the pulmonary artery. The transverse aortic arch may be reached through this incision, although it may require a cervical extension. In order to fully control the innominate artery or the right subclavian artery or vein, the median sternotomy is extended to include a right cervical incision. Extension of the sternotomy into a left cervical incision will provide access to the left common carotid artery. The mid and distal left subclavian artery and vein are more challenging to access and require a left anterolateral thoracotomy in the third or fourth interspace coupled with a left supraclavicular incision (Figure 45-5). Connecting these incisions with a vertical sternotomy is the trapdoor incision, which is discussed often but associated with significant morbidity and risk of causalgia. The descending aorta can be reached, as previously mentioned, through a left fourth interspace anterolateral thoracotomy, but preferentially should be exposed through a posterolateral thoracotomy in the same interspace. Finally, the distal pulmonary veins or the pulmonary hilum can be reached through a posterolateral thoracotomy on the appropriate side.
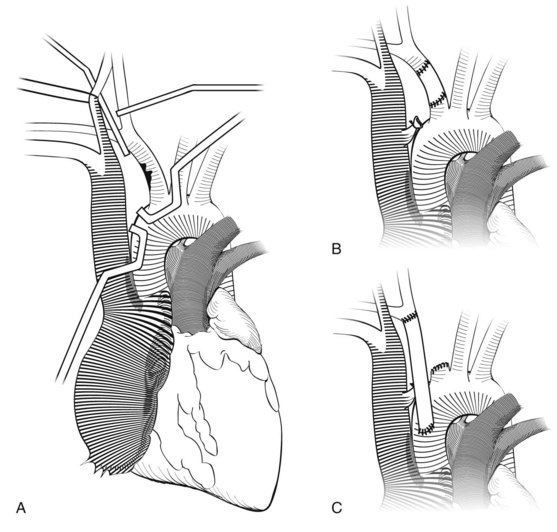
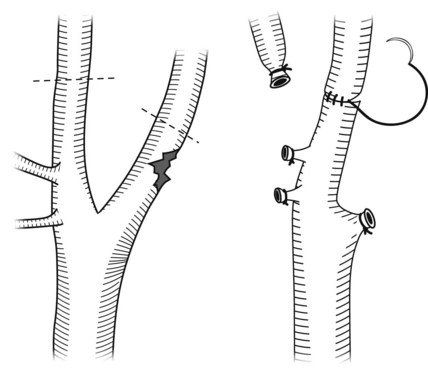
Injury to the innominate artery from penetrating trauma can occur anywhere along its length. If possible, proximal and distal control through appropriate incisions should be secured and then debridement and repair versus interposition grafting can be accomplished. Injury to the proximal innominate artery is essentially an injury to the ascending aorta (Figure 45-6). This artery is also the most common site of injury for blunt innominate artery trauma (see Figure 45-6) that is essentially an ascending aortic injury. The bypass and exclusion technique is useful in this scenario (Figure 45-7). Before entering the hematoma, the distal innominate artery just proximal to the bifurcation is controlled, clamped, and divided. Collateral flow to the carotid artery is maintained via retrograde flow from the subclavian artery, which fills through collaterals. A prosthetic graft is anastomosed to the distal innominate artery. A side-biting Satinsky clamp is placed on the aorta proximal to the origin of the innominate artery, and the graft is anastomosed to the aorta. Finally, the hematoma is entered, the injury is identified, the proximal innominate artery is divided, and the side hole in the aorta is oversewn. If there is a concomitant venous injury, a pericardial patch should be placed between the graft and the venous repair to avoid erosion and fistula formation.
Blunt Aortic Injury
The most common blunt injury in the thoracic cavity is blunt aortic injury (BAI). It was first described by Loren Parmley, a pathologist at Walter Reed Army Medical Center, in a series of 275 autopsies reported in 1958. The mortality in this series was staggering; 14% survived until arrival at the hospital, and 33% of these patients died within 24 hours, 60% by 1 week, and 90% by 4 months.65 Only 5% of these patients were considered cured.66 While there were isolated reports of attempts at repair of these lesions during that era, the state of the art was sufficiently primitive that Steinberg made a “plea for conservative treatment” of these patients.67 These injuries continue to have significant on-scene mortality, with 80% to 90% of victims not surviving the initial insult. In an analysis of the National Trauma Databank, the incidence of BAI was 0.3% of all trauma admissions and represented 8000 deaths per year. Four percent of patients were deceased on arrival to the emergency department, and an additional 19% died during triage efforts.68 Operative mortality has historically ranged from 15% to 28%,69 and paraplegia complicated nearly 9% of cases70 before the advent of alternative treatment modalities. In the initial report by Parmley and colleagues,65 57% of cases of BAI were secondary to automobile crashes, and 16% were due to airplane crashes. This remains true today; more than 80% of BAI is due to automobile accidents. In the initial report of the American Association for the Surgery of Trauma multicenter trial of BAI (known as AAST I), patients presented in one of four groups. Eight percent of patients arrived in extremis and suffered 100% mortality, all from rupture of their aorta. The other high-mortality group (9%) were those who arrived in stable condition but suffered rupture of the aorta before surgical repair; again, mortality was 100% in this subset. The most common presentation was patients in stable condition (76%) with a mortality rate of 14%, the majority of the deaths being due to nonvascular injuries. Finally, 7% of patients did not undergo operative repair; mortality in this cohort was 52% because the severity of other injuries, primarily central nervous system trauma.70 Patients with BAI typically have additional injuries to other organ systems. Closed head injury is seen in 30% to 50% of patients, multiple rib fractures in 46%, pulmonary contusion in 38%, major abdominal trauma in 29%, and pelvic fracture in 31%. The mean injury severity score (ISS) on arrival in patients with BAI is 42.68,70
The most common finding on plain film chest radiography, by far, is a widened mediastinum, seen in 85% of cases. Other findings include an indistinct aortic knob (24%), a left pleural effusion (19%), and an apical cap (19%). Uncommon findings include fracture of the first or second rib, tracheal deviation, a depressed left bronchus, and nasogastric tube deviation. No radiographic abnormalities are seen, however, in 7% of patients.70 These cases are likely due to minimal aortic injury, defined as an intimal flap less than 1 cm in size not penetrating to the media. This finding was detected in 8% of Parmley’s autopsy cases.66
Aortography was the most common diagnostic modality in the AAST I study, with transesophageal echocardiography used in a small subset of patients. However, both of these modalities have been supplanted by MDCTA in the updated report by the AAST, known as the AAST II study. Aortography decreased from 87% to 8% of cases, echocardiography from 12% to 1%, with CTA increasing from 35% to 93% of cases.71 MDCTA now stands as the modern gold standard diagnostic modality for BAI.72
In his autopsy series, Parmley described six types of BAI. The first was an intimal hemorrhage, and the second was an intimal hemorrhage with a laceration. More severe injuries include medial laceration, complete aortic laceration, pseudoaneurysm, or periaortic hemorrhage65; 93% of these injuries occur at the ligamentum arteriosum.72
In the AAST I trial, the standard of care for treatment was operative repair if the patient could tolerate the procedure, versus expectant management for those with severe multisystem injury. Clamp and sew was the usual operative approach, and mortality and paraplegia rates were 31% (22% excluding patients presenting in extremis) and 9%, respectively.70 A paradigm shift has taken place in the last decade, however. Modern treatment of BAI follows three principles. The first is aggressive pharmacologic blood pressure control, the second is appropriate selection and prioritization of repair, and the final is the use of endovascular techniques in an increasingly broad set of indications.
Antihypertensive therapy has been shown to decrease the rate of in-hospital aortic rupture from 12% to less than 2%.70,73 Intravenous esmolol or labetalol is used to titrate systolic blood pressure to approximately 100 mm Hg with a relative bradycardia. Nitroprusside can be added if additional blood pressure control is needed. While there has been a substantial reduction in the use of pulmonary artery catheters in the last decade, these patients could potentially benefit from catheter-guided titration of pressures and SvO2. Head-injured patients should be considered for intracranial pressure monitoring to optimize cerebral perfusion pressure.66
Prioritization of repair requires a balancing of the degree of injury, the risk of rupture, the ability to aggressively control blood pressure, and the risk to the patient. At one end of the spectrum, minimal aortic injury can be safely followed nonoperatively. In their series of nine patients with minimal aortic injuries, one patient underwent immediate operative repair and two died of other injuries. Of the six remaining patients, three resolved completely. One underwent operative repair demonstrating a healed flap with intimal thickening. The other two were followed clinically.73a They concluded that minimal aortic injury does not require operative intervention but should be followed until the injury has healed to rule out progression to a pseudoaneurysm.
Patients with more significant injuries should undergo repair, but their intervention can be delayed if aggressive antihypertensive therapy is instituted. Hemmila and colleagues73 showed that surgical delays beyond 16 hours did not increase mortality if a strict antihypertensive regimen was followed, but the price was increased intensive care unit and ventilator days. At the University of Michigan, the indications for delayed procedures includes significant associated injuries preventing thoracotomy and single lung ventilation, significant traumatic brain injury preventing use of heparin, other contraindications to anticoagulation, or active infection.74
Descending thoracic aortic repair of BAI is still considered by some authors to be the gold standard,74 although others would argue that endovascular stent-grafts have supplanted descending thoracic aortic repair as the mainstay of therapy, despite the lack of level I data.69 Historically, a rapid clamp-and-sew has been promoted as the most straightforward repair, although some authors believe that paraplegia rates are higher with this technique than using bypass. A multivariate analysis of risk factors for paraplegia found cross-clamp times greater than 30 minutes and the clamp-and-sew technique to be independently predictive of paraplegia postoperatively.70 One group proposes the following principles to avoid this dreaded complication: preservation of intercostal vessels intraoperatively when possible, avoidance of the clamp-and-sew technique, use of permissive hypertension postoperatively to improve spinal blood flow, and the use of spinal drains only in highly selected patients.74
Cervical Vascular Injury
Blunt Cervical Vascular Injury
Incidence and Mechanism of Injury
Several decades ago, blunt cervical vascular injury (BCVI), encompassing injuries to the carotid or vertebral arteries, was thought to be a rare injury relegated to the level of case reports. However, during the 1990s, pioneering work from trauma groups in Memphis and Denver uncovered a much larger incidence of this type of injury than had been previously described. One of the initial reports evaluated 20,349 trauma admissions at the Presley Regional Trauma Center; 67 patients (0.33%) were found to have 87 BCVIs.75 In a pilot study of all patients undergoing postinjury thoracic aortograms performed at the University of Colorado, 3.5% of patients were found to have a documented BCVI, only half of whom manifested symptoms.76 Disconcerted by these findings, the trauma surgeons in Denver undertook a prospective study in 249 patients: 40 with symptoms consistent with BCVI and 209 with high-risk features (Table 45-1). This report documented BCVI in 28 (70%) of the symptomatic patients and 57 (27%) of the asymptomatic patients. Injuries included 65 isolated carotid artery injuries, 10 isolated vertebral artery injuries, and 10 combined carotid and vertebral artery injuries.77 In another report from the same year, their overall incidence was reported at 0.38% of trauma admissions, but was 1.07% among admissions during the time they performed aggressive screening.78 Contemporary estimates of BCVI incidence is 0.4% to 1.0% of blunt trauma admissions.79–81 The mechanism of the majority of these injuries is motor vehicle crash, reported in 45% to 82% of series, followed by pedestrian trauma (12%), motorcycle crash (11%), falls (9% to 10%), and assaults (5% to 6%).75,77,79 Fifty-five percent of patients with BCVI had a lateralizing examination in the University of Michigan series. In the 45% without a lateralizing examination, neurologic findings became evident from 3 hours to 10 days after admission.79
TABLE 45-1 Screening Criteria for Blunt Cervical Vascular Injury
| Hard Signs of BCVI | Signs Suggestive of Occult BCVI |
|---|---|
| Hemorrhage from the mouth, ears, nose, or wounds | Severe cervical hyperextension and rotation or hyperflexion if associated with displaced midface or complex mandibular fracture or closed head injury consistent with diffuse axonal injury |
| Expanding cervical hematoma | Near hanging resulting in anoxic brain injury |
| Cervical bruit in a patient <50 years old | Seat belt abrasion or other soft tissue injury of the anterior neck resulting in significant swelling or altered mental status |
| Evidence of cerebral infarction on CT scan | Basilar skull fracture |
| Unexplained or incongruous neurologic deficit, transient ischemic attack, amaurosis fugax, or Horner syndrome | Cervical vertebral body fracture |
BCVI, Blunt cerebrovascular injury; CT, computed tomography.
From Biffl WL, Moore EE, Offner PJ, et al: Blunt carotid arterial injuries: implications of a new grading scale. J Trauma 47:845–853, 1999.
Screening
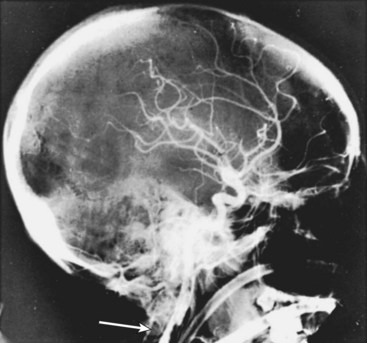
Given the unexpectedly high incidence of BCVI in blunt trauma, investigators began searching for a means of detecting the injury in asymptomatic patients. In the original report from Memphis, circumstances that prompted invasive evaluation with cerebral angiography included cervical soft tissue injury, a neurologic examination incompatible with imaging findings, the development of a neurologic deficit after admission, or the presence of Horner syndrome.75 In an effort to further elucidate which patients were at risk, the Denver group developed the following criteria. Patients underwent four-vessel cerebral angiography (FVCA) for any of the following: hemorrhage from the mouth, ears, nose, or wounds; expanding cervical hematoma; cervical bruit in a patient younger than 50 years; evidence of cerebral infarction on CT scan, or an unexplained or incongruous neurologic deficit, transient ischemic attack, amaurosis fugax, or Horner syndrome. The group would also screen patients with FVCA for the following: severe cervical hyperextension and rotation or hyperflexion if associated with displaced midface or complex mandibular fracture or closed head injury consistent with diffuse axonal injury; near hanging resulting in anoxic brain injury; or a seat belt abrasion or other soft tissue injury of the anterior neck resulting in significant swelling or altered mental status, basilar skull fracture, or cervical vertebral body fracture.77 In an effort to standardize practice, the Eastern Association for the Surgery of Trauma (EAST) published recommendations for the screening, diagnosis, and treatment of BCVI. They found no level I evidence. Level II recommendations are to screen for any neurologic abnormality not explained by a diagnosed injury, or blunt trauma associated with epistaxis from suspected arterial source. Level III evidence supports screening asymptomatic patients with blunt head trauma and the following risk factors: Glasgow Coma Scale score of 8 or less, petrous bone fracture, diffuse axonal injury, cervical spine fracture (especially C1-C3 and through foramen transversarium or with subluxation or rotational component), or a Lefort II or III facial fracture.82 The major hurdle at this point, and the reason behind the efforts to elucidate an at-risk population, is that FVCA still remains the gold standard for diagnosis. The EAST guidelines state that duplex ultrasonography and four-slice or less CT scanners are inaccurate for the detection of BCVI. FVCA is, however, labor intensive, expensive, and invasive. There is a great deal of enthusiasm for the latest generation of MDCTA systems (MDCTA).77 However, a report from the Memphis group raised a cautionary flag after they found an overall 51% sensitivity with MDCTA in a thorough, prospective study in which all patients underwent both MDCTA and FVCA.81 At this time, FVCA seems to be the most conservative, safe option for at-risk patients (Figure 45-8).
Findings and Outcomes
The Denver group77 proposed a grading scale for BCVI in 1999 (Table 45-2). The scale correlates well with mortality and stroke risk and has been used as the basis for treatment recommendations. In a recent report, the overall stroke risk for patients with BCVI was 14% and the in-hospital mortality was 10%.81 Another report cited 26% in-hospital mortality. Among those who survived to discharge, only 31% were discharged home, whereas 43% were transferred to another inpatient facility such as rehabilitation or skilled nursing.83 By injury grade, mortality was 0% to 33% for grade I-III lesions, 11% to 60% for grade IV, and 100% for grade V; stroke risk was 3% for grade I, 11% for grade II, 33% for grade III, 44% for grade IV, and 100% for grade V.78,79,84,85 These findings agree with the early report from the Memphis group, which documented an overall 31% mortality among BCVI patients. They also noted that the mortality was 100% in patients not treated with systemic heparin and that all these deaths were due to stroke, compared to a 20% mortality for those treated with heparin.75 The location of the lesions was primarily in the cervical internal carotid artery (68%) in the Michigan series; skull base carotid injury occurred in 45%, and intracranial injury occurred in 32%.79
TABLE 45-2 Grading Scale for Blunt Carotid Injury
| Injury Grade | Description |
|---|---|
| I | Luminal irregularity or dissection with <25% luminal narrowing |
| II | Dissection or intramural hematoma with ≥25% luminal narrowing, intramural thrombus, or raised intimal flap |
| III | Pseudoaneurysm |
| IV | Occlusion |
| V | Transection with free extravasation |
From Biffl WL, Moore EE, Offner PJ, et al: Blunt carotid arterial injuries: implications of a new grading scale. J Trauma 47:845–853, 1999.
Treatment of Blunt Cerebrovascular Injury
The primary treatment for BCVI is anticoagulation. Since the report by Fabian and colleagues75 in 1996 demonstrated that heparin was the only factor independently predictive of improved neurologic outcomes, anticoagulation has been considered appropriate therapy for most BCVI, with surgery or endovascular treatments considered for specific lesion types. Recommendations from EAST are that heparin should be started without a bolus and should be converted to warfarin (Coumadin) with a goal initial normalized ratio (INR) of 2 to 3 for a period of 6 months.82 No PTT goal is given in the EAST recommendations, but other authors have recommended a conservative goal of 40 to 50 seconds, especially in patients at high risk for bleeding.75 There is also literature supporting the equivalence of antiplatelet therapy to anticoagulation in this patient population; in a study of 22 patients with BCVI, there were no differences in neurologic outcome among patients treated with anticoagulation or antiplatelet therapy, but there was a significant decrease in bleeding events with antiplatelet agents.79 The other general recommendation from EAST is that patients with an early neurologic deficit and an accessible lesion should undergo operation or intervention to restore flow as soon as possible.82
Treatment of Grade I Injury
A grade I injury, defined as a luminal irregularity or dissection with less than 25% narrowing of the lumen, is thought to result from stretching of the artery or a direct blow. The pathophysiology is similar to that which produces Horner syndrome, a stretch or blow that disrupts the sympathetic ganglion fibers. Exposure of the thrombogenic subendothelial collagen puts patients at risk for thromboembolic strokes, which occur in approximately 3% of these patients.78 Over time, 62% of nonocclusive dissections resolve with anticoagulation,75 which supports the EAST recommendations that these patients be treated with antiplatelet or anticoagulation therapy.82 However, there are no prospective data supporting the type or duration of therapy, and treatment should be tailored to the patient, the lesion, and comorbid injuries.
Treatment of Grade II Injury
Grade II injury—defined as a dissection or intramural hematoma with luminal narrowing of 25% or more, intramural thrombus, or raised intimal flap—has a 70% risk of progression to grade III or IV despite full anticoagulation; only 10% of these wounds heal. Despite this progression to a higher grade lesion, anticoagulation seems to be protective against stroke and is recommended. It is also recommended that these patients have repeated imaging studies 7 days after the injury to evaluate for progression.78,82
Treatment of Grade III Injury
Grade III injuries are pseudoaneurysms that develop secondary to egress of blood into the subadventitial layer and often result from progression of grade II injuries. Pseudoaneurysms pose a risk for rupture and hemorrhage, as well as thromboembolism; they rarely resolve with anticoagulation.75,78 If the aneurysms are surgically accessible, operative repair is appropriate, although the distal artery may be surprisingly friable and difficult to work with. If the lesion is not surgically accessible, stenting is becoming a preferred treatment. One report initially warned of a 45% occlusion rate following carotid stenting, but these patients were treated with warfarin therapy as opposed to antiplatelet therapy, which has become standard of care for patients with stents until endothelialization is complete.86 No occlusions were detected by Edwards and colleagues in stented arteries when patients were treated with clopidogrel.83 The acutely injured artery, however, should not be subjected to angioplasty or stenting within 48 to 72 hours after injury.78 Consensus opinion seems to be that patients with grade III injuries should be initially given anticoagulants and reimaged at 7 days after the injury. If the pseudoaneurysm persists (or has developed), consider stent placement with or without coil embolization and convert the patient to antiplatelet therapy; heparin and warfarin therapy may be continued or discontinued at the discretion of the surgeon based on the patient’s overall risk and catalog of injuries. One final option for high skull-based lesions is an extracranial-intracranial bypass; this is a specialized skill requiring a microvascular neurosurgeon, however, and is not usually available at smaller hospitals.
Treatment of Grade IV and V Injury
Grade IV lesions are occlusions, and grade V injuries are transections. Both are highly morbid and lethal injuries. Some authors believe that outcomes are generally independent of treatment efforts,79 although others have suggested decreased stroke risk with heparin therapy for grade IV lesions.78 Interestingly, one center reports treating occlusion of the internal carotid artery in asymptomatic patients after penetrating trauma with embolization to prevent an occluded artery from “reopening” and providing a substrate for distal embolization after vasospasm dissipates.26 Grade V transections require obtaining proximal and distal control, which can be extremely challenging depending on the location of the injury. Outcomes are miserable; one series reported 100% mortality from this injury.78
Penetrating Cervical Vascular Injury
Incidence, Mortality, and Mechanism of Injury
Penetrating injury to the cervical vessels has historically been treated somewhat differently than BCVI, because of differences in pathophysiology, diagnosis, treatment, and outcomes. In a series of 63 patients with combat-trauma–related penetrating neck trauma with suspected vascular injuries, 21 patients underwent ligation or repair in-theater. All patients underwent further evaluation following evacuation, which revealed 12 additional occult injuries and one graft thrombosis that were subsequently repaired. Blast fragments were responsible for the majority of these wounds (79%), with high-velocity gunshot wounds being responsible for the remainder.87 In civilian trauma, the most common mechanism is a gunshot wound.20 In a series of 38 civilian gunshot wounds to the internal carotid artery (ICA), 89% of patients had symptoms including neck hematoma (84%), active hemorrhage (32%), and neurologic deficits (26%). Mortality in this series was 19%, primarily because of stroke. The most common associated injury was pharyngeal (39%), which has implications for management of the suture line. The most commonly injured associated vascular structures were the internal maxillary artery (16%), external carotid artery (11%), and vertebral artery (8%). The likelihood of ICA occlusion correlated with the location of injury along the ICA; 20% of injuries of the ICA origin led to occlusion, compared to 29% of injuries below the odontoid process, 73% at the odontoid process, and 100% at the skull base.26 Rao and colleagues88 evaluated 76 patients with cervical vascular injuries among 528 patients with penetrating neck trauma. The overall mortality rate was 2.5%; among those with vascular injury, the mortality rate was 16%. Four patients underwent therapeutic embolization of an injured vessel in zones 1 or 3.88
Zones of the Neck
In 1969, Monson and colleagues89 described three zones of cervical trauma with implications for technique of exposure and exploration. Zone 1 encompasses the root of the neck and is defined by the clavicles inferiorly and the cricoid cartilage superiorly. Zone 2 is the midcervical region defined by the cricoid cartilage inferiorly and the angle of the mandible superiorly. Zone 3 is superior to the angle of the mandible.89
Diagnosis
Classically, surgical exploration is the diagnostic maneuver used for a zone II wound with hard signs of vascular injury such as intraoral or extraoral bleeding, an expanding hematoma, a palpable thrill or audible bruit, or the loss of carotid pulse with a neurologic deficit. Soft signs in this region, such as a wound with proximity to the carotid artery, a stable hematoma, a carotid-jugular fistula at an unknown level, or the loss of the carotid pulse without a neurologic deficit, should undergo imaging to delineate the injury. It is important to remember that high-energy penetrating missiles can cause blunt injury resulting from cavitation in addition to direct trauma. This type of injury must be considered and treated appropriately. Similarly, zone I injuries should undergo arteriorraphy both for diagnosis and operative planning. Zone III injuries are diagnosed with arteriorraphy, which can present therapeutic solutions at the same time if the lesion is in a location not easily surgically accessible.90 The ability of MDCTA to detect BCVI has been questioned recently; however, MDCTA seems to have better sensitivity for penetrating cervical injury in select patients. Fox and colleagues87 found that the presence of nearby fragments decreased the sensitivity of this modality and recommended checking a plain film first if MDCT-A is planned. FVCA remains the gold standard. Duplex examination is often limited by the wound, dressing, or injuries to the cervical spine.
Management
The first issue to address with penetrating cervical trauma is airway management. In addition to the usual maneuvers, there are special considerations for active bleeding involving the airway. Proximal compression of the common carotid can be helpful to control bleeding while establishing a protected airway. Intraoral bleeding may be temporized with finger pressure, balloon occlusion, or gauze packing. A contained cervical hematoma may deviate the trachea and elevate the floor of the mouth, making orotracheal intubation challenging or impossible. Nasotracheal intubation is an option if the correct instruments are available, but cricothyroidotomy should be performed expeditiously if it is unavailable or unsuccessful.90
There are several damage control options available in the cervical region. Rapid insertion of a balloon catheter such as a Foley into the wound tract, inflation, and outward traction may provide tamponade and temporize bleeding. In zone III, exposure is generally difficult and the ICA may be difficult to compress. One option is to perform an arteriotomy on the common carotid after controlling it with vessel loops, insert a no. 3 or 4 Fogarty embolectomy catheter, and advance and inflate it in a serial fashion until bleeding from the distal ICA abates. This may also be done using angiographic guidance if the patient is in the fluoroscopy suite. An additional advantage of intramural balloon occlusion is that a determination of patient tolerance to ligation can be determined. If the patient manifests a focal neurologic deficit with occlusion, some form of repair should be attempted if possible. Finally, for resistant bleeding high in zone III, packing of the carotid canal with bone wax may allow tamponade for life-saving purposes.26,90 After hemostasis is achieved with a damage control strategy, the options include: formal high cervical exploration for repair or ligation; arteriographic embolization and stenting; or 48-hour balloon occlusion. The Fogarty balloon is filled with contrast and the patient is monitored for signs of cerebral ischemia. If there is no sign of ischemia, the balloon remains in place until 48 to 72 hours after placement, at which time it is deflated and removed. The patient should be imaged with a crossover angiogram to rule out a pseudoaneurysm above the occlusion. If the patient manifests signs of ischemia, a bypass from the cervical ICA to the petrous ICA is necessary and will require the assistance of a neurosurgeon.91
The types of injuries that can be present with penetrating trauma are similar in type and recommended treatment to those for BCVI, although major wall disruption is much more likely. For intramural hematomas, intimal defects, or dissection with preserved distal flow, treatment similar to that for grade I and II BCVI is appropriate, namely heparinization. Stenting may be appropriate to tack down the intima for major dissections. Patients should undergo repeated imaging in 1 to 2 weeks. Pseudoaneurysm with contrast extravasation should be either observed (for small aneurysms) or stented with or without coil embolization. Heparin is risky in this situation given the possibility of rupture90; however, antiplatelet therapy should be used if a stent is placed. For occlusions, anticoagulation is recommended to prevent distal propagation or embolization. As previously mentioned, embolization of the thrombosed ICA is recommended.26 Finally, carotid-jugular arteriovenous fistulae may be asymptomatic if small, but if sufficiently large they can produce high-output heart failure or coma secondary to elevated intracranial pressure. Treatment is with operative repair for lesions in zone II and embolization in zone III.26,90
There has historically been controversy regarding the surgical management of the patient with a cervical vascular wound and a neurologic deficit, with concern that revascularization would convert an area of ischemia into hemorrhage. This issue was addressed in 1978 by Liekweg and Greenfield.92 Among noncomatose patients, 85% of patients with neurologic deficits who are revascularized have positive outcomes versus 50% of those ligated (p < 0.05). However, among comatose patients, favorable outcome with repair was seen in 27% versus 25% of patients undergoing ligation (p = not significant).92 Current practice is to revascularize all patients with penetrating injury regardless of neurologic deficits except for coma. Patients with Glasgow Coma Score less than 8 not related to hypovolemia, hypothermia, or intoxication are likely to have an adverse outcome regardless of management. If the presentation is delayed more than 3 to 4 hours after injury, a CT scan should be obtained. If an ischemic stroke is present, revascularization is contraindicated, because this may convert to a hemorrhagic infarct.90
Exposure of these wounds varies by zone of injury. The simplest is zone II, which can be approached with a standard anterior sternocleidomastoid incision similar to that used for a carotid endarterectomy. Injuries to zone I may require thoracotomy or sternotomy to access the aortic arch and the roots of the great vessels for proximal control. Zone III injuries necessitate a high skull-base dissection. Adjuncts such as mandibular subluxation or osteotomy are occasionally necessary to approach the vessels surgically, and therefore endovascular repairs are ideal if possible in this position. Before clamping the vessel, patients should be systemically heparinized with 100 U/kg. Local heparin flushes with 50 U/mL concentration should be used to irrigate locally to ensure vessels are cleared of thrombi.90 Patients should also be given anticoagulants if observation is chosen; repeated imaging is mandatory.
Surgical options include ligation, primary repair, bypass graft with autogenous vein (Figure 45-9) or prosthetic graft, patch graft, or external carotid to internal carotid bypass for injuries near the carotid bulb (Figure 45-10). As mentioned previously, Liekweg and Greenfield92 demonstrated improved outcomes for revascularization over ligation for noncomatose patients. For comatose patients, they recommend repair only if there is evidence of prograde flow.92 If bypass grafting is performed, polytetrafluoroethylene (PTFE) is appropriate for common carotid injuries; autogenous vein should be used for ICA repairs given the improved patency rates.90 As always, prosthetic grafts should be avoided if possible in contaminated wounds.87 If surgical repair is performed, a careful search should be made for injury to other structures, especially the aerodigestive tract (Figure 45-11). If such an injury is encountered, in addition to repairing it appropriately, the vascular suture line should be isolated by means of interposition of vascularized tissue. The sternocleidomastoid muscle can be detached from its sternal head and rotated to cover the repair. Nonoperative therapy options include angioplasty to tack down intimal flaps and coil embolization and stents for pseudoaneurysms. In patients where observation is selected as the treatment plan, repeated imaging is necessary to ensure resolution of the lesion. In the series by Fox and colleagues,87 vertebral artery occlusions were safely observed.
Abdominal Vascular Injury
Almost all cases of abdominal vascular injury are caused by penetrating trauma, usually from a gunshot wound. The AAST grading system for abdominal vascular injury is shown in Table 45-3. Associated damage to gastrointestinal organs is common, yielding a lethal combination with an associated mortality rate greater than 50%. One of the problems leading to a high mortality rate is that the symptoms are often not specific. Hypotension and abdominal distension are commonly seen, and distal pulse deficits may be appreciated. In planning the exploration of potential abdominal vascular injury, it is best to have sufficient large-bore intravenous access already established, blood or blood products in the room, and the anesthesia team fully prepared for an unstable patient. If there is any sign of renal vascular or parenchymal injury, such as hematuria or an injury tract near the kidneys, a one-shot intravenous pyelogram may be helpful to confirm the presence of a contralateral functional kidney if a nephrectomy becomes necessary.
TABLE 45-3 American Association for the Surgery of Trauma–Organ Injury Scale for Abdominal Vascular Injury
| Grade | Description |
|---|---|
| I | Unnamed superior mesenteric artery or superior mesenteric vein branches; unnamed inferior mesenteric artery or inferior mesenteric vein branches; phrenic artery or vein; lumbar artery or vein; gonadal artery or vein; ovarian artery or vein; other unnamed small arterial or venous structures requiring ligation |
| II | Right, left, or common hepatic artery; splenic artery or vein; right or left gastric arteries; gastroduodenal artery; inferior mesenteric artery, trunk, or inferior mesenteric vein, trunk; primary named branches of mesenteric artery (e.g., ileocolic artery) or mesenteric vein; other named abdominal vessels requiring ligation or repair |
| III | Superior mesenteric vein, trunk; renal artery or vein; iliac artery or vein; hypogastric artery or vein; vena cava, infrarenal |
| IV | Superior mesenteric artery, trunk; celiac axis proper; vena cava, suprarenal, and infrahepatic; aorta, infrarenal |
| V | Portal vein; extraparenchymal hepatic vein; vena cava, retrohepatic, or suprahepatic; aorta, suprarenal, subdiaphragmatic |
This classification system is applicable for extraparenchymal vascular injuries. If the vessel injury is within 2 cm of the organ parenchyma, refer to specific organ injury scale. Increase one grade for multiple grade III or IV injuries involving >50% vessel circumference. Downgrade one grade if 25% vessel circumference laceration for grades IV or V.
Abdominal Aortic Injury
The aorta is the most commonly injured vessel in the abdomen. Historically, this injury was rarely seen, primarily because patients would not survive to medical care after sustaining aortic trauma. In World War I, Makins reported five cases of aortic injury in the British experience (0.4% of all vascular injuries reported) with a 100% mortality.92a This improved slightly in World War II; three aortic injuries (0.1% of vascular trauma) were reported with a 67% mortality.2 In a series of 470 patients with abdominal vascular injuries reported by Tyburski et al, there were 71 injuries to the aorta (15% of patients); 62% of these injuries involve the pararenal or suprarenal segments of the aorta, which are associated with 92% mortality, compared to 66% in the infrarenal segment.93 The overwhelming majority of injuries to the aorta are from penetrating trauma, primarily gunshot wounds. Among the approximately 10% of aortic injuries from blunt trauma, motor vehicle crashes are the usual mechanism.29,94,95
Patients with abdominal aortic injury are usually in hypovolemic shock; the lethal triad of hypothermia, acidosis, and coagulopathy is commonly seen. Some authors are aggressive with EDT in these patients, but the results are poor and others recommend avoiding EDT in patients with abdominal vascular injury.94 Clues to the location of injury can be gleaned from the physical examination. Injury above or involving the SMA will produce abdominal pain, pararenal involvement may lead to hematuria, or injury to the infrarenal aorta may manifest as unilateral or bilateral lower extremity ischemia.
Among patients who survive the initial operation, primary suturing is the most common repair technique (69% to 86%), followed by prosthetic graft in 14% to 23%; 17% to 32% of patients do not survive the operation.94,95 Small wounds of the aorta can be closed primarily with 3-0 or 4-0 polypropylene sutures. If there are multiple aortic wounds in close proximity, they can be connected surgically to allow for a single closure. If possible, the wound should be closed transversely to prevent stenosis, although up to a 50% luminal narrowing is generally well tolerated and may be addressed at a later date.96 If there is significant tissue loss to the aortic wall, patch aortoplasty with PTFE or interposition grafting with 12- to 16-mm Dacron woven grafts may be necessary.
Concomitant hollow viscus injury and field contamination is a common problem in aortic trauma.97 One technique for managing this injury is to use a Dacron graft, as the literature does not support a high-risk of graft infection in young trauma patients. It is wise to thoroughly irrigate the abdomen and protect the graft with an omental pedicle if interposition grafting is performed. The alternative is ligation and delayed extraanatomic bypass.29 The obvious downside of this approach is the need for two general anesthetic cases, as well as an iatrogenic ischemia-reperfusion insult.
In cases of blunt aortic injury, endovascular strategies may be useful. Chronic dissections are best treated by rigid, balloon-expandable stents, whereas self-expanding stents are better tolerated for acute dissections. The obvious advantage of this approach is the avoidance of a retroperitoneal dissection, aortic cross-clamping, and prevents contamination of vascular graft materials in cases of contaminated fields. Yeh and colleagues98 describe a case report of a ruptured mycotic pseudoaneurysm treated successfully with endovascular exclusion 14 days after the initial injury and primary aortic repair. The field had been contaminated because of multiple enterotomies, leading to the mycotic pseudoaneurysm. This approach avoided a tedious, dangerous dissection through a hostile abdomen in a hemorrhaging patient; the patient survived.98
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree