Vascular Masses of the Mediastinum
Alberto de Hoyos
Ali Dodge-Khatami
Carl L. Backer
Mediastinal lesions of vascular origin are responsible for approximately 10% of mediastinal masses. They may mimic neoplasms and must be considered in the differential diagnosis of mediastinal masses. Knowledge of the vascular anatomy of the mediastinum along with its variations and anomalies should suggest the vascular origin and guide appropriate diagnostic imaging and treatment.102 Vascular masses of the mediastinum may be categorized according to one of several methods. They can be classified according to the primary compartments of the mediastinum from which they arise: anterior, visceral, and paravertebral. However, because most vascular masses originate in the visceral compartment, a more useful classification of mediastinal masses of vascular origin is that suggested by Kelley and associates.90 They classified mediastinal vascular masses as arising from either the pulmonary or systemic circulation and as being either arterial or venous in origin. In the direction of flow, starting at the confluence of the systemic innominate veins, they are as follows: systemic venous system, pulmonary arterial system, pulmonary venous system, and systemic arterial system.174,175
From a practical standpoint, however, most masses are initially recognized as abnormalities on plain chest radiogra- phics.32,174,175,181 Bousmara21 suggests that a differential diagnosis may be established by considering the radiographic region involved and the possible vascular structures contained therein or nearby (Table 176-1). Confirmation of the diagnosis is usually made by contrast-enhanced computed tomography (CT), magnetic resonance imaging (MRI), or echocardiography.50,51,79,116,117,129,149,151,155,178,179 McAdams112 and Gilkeson54 have demonstrated the value of these studies in the diagnosis of mediastinal hemangiomas and lesions of the aorta, respectively. For patients with complex lesions and those with concomitant cardiac abnormalities, biplane cardiac cine angiograms are often necessary. MRI, because of its noninvasive nature, is the imaging modality of choice for further evaluation following CT angiography. Conventional angiography is now rarely necessary and is not routinely recommended.
Systemic Venous System
Anomalies of the systemic venous system causing a mediastinal vascular mass include aneurysms of the innominate vein and superior vena cava (SVC), dilatation of the SVC associated with partial anomalous venous return, persistent left SVC, and azygos or hemiazygos enlargement. The diagnosis of these lesions is often suggested by plain radiographs, but frequently CT angiography or MR venography is necessary for confirmation.57,67,68,94,126,140,166,176,179
Vena Cava and Tributaries
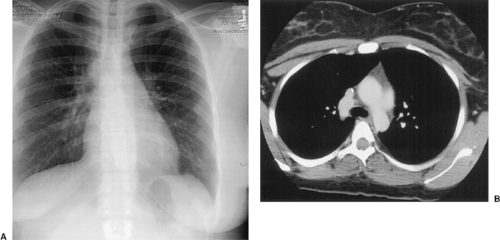
The great majority of cases of dilatation of the SVC are the result of elevated central venous pressure, commonly from cardiac decompensation and less often from tricuspid stenosis or cardiac tamponade secondary to pericardial effusion or constrictive pericarditis. Other common causes are listed in Table 176-2. The radiographic appearance is distinctive and consists of a smooth, well-defined widening of the right side of the mediastinum. The azygos vein is almost always dilated as well, a finding that is a more dependable sign of systemic venous hypertension, because the diameter of the vein in the right tracheobronchial angle can be precisely measured. Rarely, the SVC has an aneurysmal dilatation (Fig. 176-1). Aneurysm of the SVC is extremely rare, with <30 cases described in the English literature.94 Most are asymptomatic and are discovered incidentally as widening of the upper mediastinum, simulating a paramediastinal mass, on chest radiographs. These lesions are better visualized on expiration or with the patient supine, as this maximizes aneurysm volume. Aneurysms of the SVC can be classified as either fusiform or saccular and can reach >10 cm in diameter. The etiology is unknown but presumably involves congenital weakness of the SVC wall. Absence of the longitudinal muscular layer has been described.166 Management of SVC aneurysms is currently undefined and controversial. For saccular-type aneurysms, which are connected to the SVC by a narrow stalk, surgical resection is recommended owing to potential complications including pulmonary embolism, rupture, and partial or total venous compression and obstruction. In contrast, conservative management is recommended for fusiform aneurysms, as these do not enlarge, produce pressure symptoms, or rupture, and pulmonary embolism has not been reported.57,94,166 Occasionally, the inferior vena cava can also undergo localized aneurysmal dilatation and be visible as a smooth, sharply circumscribed opacity in the right cardiophrenic angle.66 Cystic hygroma of the mediastinum has been found to be associated with abnormal enlargement or aneurysmal dilatation of neck or thoracic veins. Diagnosis is usually established with contrast-enhanced CT.
Table 176-1 Vascular Lesions of the Mediastinum Based on Chest Radiographs | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
More common than an aneurysm of the SVC is dilatation of the SVC from partial anomalous pulmonary venous return from the right lung to the SVC. Generally, this occurs with a subcaval atrial septal defect and is referred to as a sinus venosus atrial septal defect. The right upper and middle lobe pulmonary veins (right superior pulmonary vein) drain into the lower portion of the SVC or the SVC–right atrial junction. In adults, a more common finding is an anomalous left-upper-lobe vein connecting to a persistent vertical vein and creating an abnormal left mediastinal contour.66
Persistence of the left SVC is a relatively common anomaly that occurs in 0.3% of normal subjects and in about 4.4% of patients with congenital heart disease; it may mimic a mass on the chest radiograph.25 Most patients with this anomaly have both a right and a left SVC, but the right SVC may be absent.7,106 Occasionally, a persistent brachiocephalic vein connects the two SVCs. Persistence of the left SVC may present on the chest radiograph as a widening of the aortic shadow, a paramediastinal bulge, a paramediastinal strip, or a crescent along the upper left cardiac border.35 It results from persistence of the left anterior and common cardinal vein and the left sinus horn. Although the diagnosis of this anomalous vessel may be suggested from plain radiographic findings, CT and MRI are virtually diagnostic.7,106,177 On CT, the left SVC is seen as a round density just to the left of the aortic arch. If contrast material is administered via the left arm, the vein enhances (Fig. 176-2). A persistent left SVC extends inferiorly to the region of the left superior pulmonary vein and then connects into the coronary sinus and has no physiologic effect. Rarely, the vein may connect directly into the left atrium, giving rise to a right-to-left shunt. It can also be associated with the unroofed coronary sinus syndrome, which also causes a right-to-left shunt.138 The shadow created by an anomalous left SVC can be simulated by that caused by anomalous venous drainage of the left upper lobe.
Aneurysms of the innominate vein are rare. They originate in the visceral compartment but project into the anterior compartment on a lateral chest film.61 On the left side of the mediastinum this may overlie the aortic knob and give the appearance of a “double” aortic knob, which may also be caused by pseudocoarctation of the aorta, from which it must be differentiated.22,164,186
Enlargement of the azygos vein can mimic a right upper mediastinal or paratracheal mass or enlarged lymph nodes (Fig. 176-3). Heitzman71 defined an enlarged azygos vein as one that is perpendicular to the right mainstem bronchus and measures >7 mm. There are many causes of dilatation of the azygos and hemiazygos veins: (a) portal hypertension, (b) anomalous pulmonary venous drainage, (c) acquired obstruction of the superior or inferior vena cava, (d) azygos continuation of the inferior vena cava, (e) persistence of the left SVC, (f) hepatic vein obstruction, and (g) absence of the retrohepatic inferior vena cava. Aneurysms of the azygos vein have also been described.1,28,45,55,76,98,123,128,131,132,153 An anomalous left brachiocephalic vein (ALBCV) can also appear as a mass lateral to the arch of the aorta. This vessel crosses the middle mediastinum behind the ascending aorta, between it and the trachea, masquerading as an aortopulmonary window mass. The vessel then enters the SVC caudal to the azygos vein. The ALBCV has a recognized association with congenital heart disease, with a reported
incidence of 0.2% at autopsy and 0.98% at echocardiography.129 It is more commonly seen with tetralogy of Fallot and ventricular septal defect with pulmonary atresia.
incidence of 0.2% at autopsy and 0.98% at echocardiography.129 It is more commonly seen with tetralogy of Fallot and ventricular septal defect with pulmonary atresia.
Table 176-2 Causes of Dilatation of the Superior Vena Cava | |
|---|---|
|
The most common cause of enlargement of the azygos vein is elevated central venous pressure as a result of cardiac decompensation, lesions of the tricuspid valve, acute pericardial tamponade, or constrictive pericarditis (Table 176-3). Radiographically, dilatation of the azygos vein is evidenced by a round or oval shadow in the right tracheobronchial angle >10 mm in diameter in the erect position and >14 mm in the supine position. Measurements exceeding these figures indicate an increase in either pressure or flow.
In plain chest radiographs, a dilated azygos vein can be differentiated from an enlarged azygos lymph node fairly easily by comparing the diameter of the shadow on radiographs exposed in the erect and supine positions. When the vein is responsible, there is a noticeable difference in size. On a chest x-ray, the distended azygos arch produces a right paratracheal mass, which should reduce in size during the Valsalva maneuver (forced expiration with closed glottis), allowing the distinction from other mediastinal masses. Azygos or hemiazygos continuation can also be mistaken for a paravertebral mass on plain film, in which case CT will confirm the underlying venous anomaly. When the nature of the shadow is in doubt, CT angiography provides definitive information. Dynamic CT may reveal marked enhancement of the mass during the late venous phase, suggesting a vascular structure. Confirmation of diagnosis can be made by MRI, using a fast gradient echo imaging technique in cine mode, showing turbulent flow in the dilated aneurysmal azygos, and contrast-enhanced MRI angiography, demonstrating a dilated azygos vein. When thrombosed, azygos vein aneurysms may appear as solid masses. Although conservative management is suggested, resection may be required to prevent complications such as pulmonary embolism or hemoptysis. Similarly, the CT appearance of azygos and hemiazygos continuation of the inferior vena cava is so characteristic that angiography is seldom indicated for diagnosis.47,172 In this condition, venous blood from the lower extremities and abdomen is diverted into the azygos or, less commonly, the hemiazygos, resulting in dilatation of these veins. Chest radiographs usually show an enlarged azygos vein and a widened paraspinal reflection; the inferior vena cava is usually not visualized on the lateral film. CT or MRI shows enlargement of the azygos or hemiazygos veins and marked dilatation of the azygos arch or the left supreme intercostal vein (Fig. 176-4). Azygos continuation of the inferior vena cava is characterized by the “candy stick” appearance of the dilated azygos vein on venography, but contrast-enhanced CT or MRI is usually diagnostic. This anomaly is often associated with a congenital cardiac malformation, with errors in abdominal situs, or with asplenia or polysplenia. Most patients are asymptomatic. Of 32 cases reviewed by Anderson,6 situs was normal in 18 and abnormal in 14. The accessory hemiazygos vein on the left side may enlarge in a fashion analogous to the azygos vein on the right side. Dilatation of the left supreme intercostal vein generally possesses the same etiologic significance as that of dilatation of the azygos vein, although radiographic visualization of this vein is not nearly as frequent as that of the azygos vein. The normal left supreme intercostal vein originates from the confluence of the second, third, and fourth left intercostal veins. As it passes
anteriorly from the spine, it relates intimately to some portion of the aortic arch. In this location it may be confused with a persistent left SVC. The supreme intercostal vein connects the left brachiocephalic vein with the hemiazygos system. It runs parallel to the aortic arch and normally measures no more than 4 mm in diameter. As noted by Haswell,69 it can become dilated, and in this location it is seen end-on as the aortic “nipple,” a local protuberance that is identifiable in about 10% of normal subjects (Fig. 176-5). On erect posteroanterior radiographs, the maximum diameter of the aortic nipple in normal subjects is 4.5 mm. A diameter greater than this is a useful sign of circulatory abnormalities, the most common of which is cardiac decompensation; other causes include (a) congenital or acquired obstruction of the vena cava, as in azygos continuation of the inferior vena cava, (b) hypoplasia of the left innominate vein, (c) portal hypertension, (d) Budd-Chiari syndrome, (e) obstruction of the inferior vena cava, and (f) congenital absence of the azygos vein. Hemiazygos continuation of the inferior vena cava is particularly associated with the heterotaxy syndrome polysplenia.52
anteriorly from the spine, it relates intimately to some portion of the aortic arch. In this location it may be confused with a persistent left SVC. The supreme intercostal vein connects the left brachiocephalic vein with the hemiazygos system. It runs parallel to the aortic arch and normally measures no more than 4 mm in diameter. As noted by Haswell,69 it can become dilated, and in this location it is seen end-on as the aortic “nipple,” a local protuberance that is identifiable in about 10% of normal subjects (Fig. 176-5). On erect posteroanterior radiographs, the maximum diameter of the aortic nipple in normal subjects is 4.5 mm. A diameter greater than this is a useful sign of circulatory abnormalities, the most common of which is cardiac decompensation; other causes include (a) congenital or acquired obstruction of the vena cava, as in azygos continuation of the inferior vena cava, (b) hypoplasia of the left innominate vein, (c) portal hypertension, (d) Budd-Chiari syndrome, (e) obstruction of the inferior vena cava, and (f) congenital absence of the azygos vein. Hemiazygos continuation of the inferior vena cava is particularly associated with the heterotaxy syndrome polysplenia.52
Table 176-3 Enlarged Azygos Vein | ||||
|---|---|---|---|---|
|
Table 176-4 Causes of Dilatation of the Main Pulmonary Artery | |
|---|---|
|
Pulmonary Arterial System
Major Pulmonary Arteries
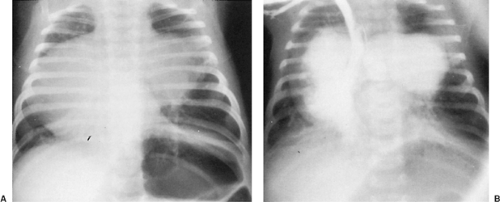
The main pulmonary artery may be so greatly dilated as to suggest a mediastinal mass. Anomalies of the pulmonary arterial system causing a mediastinal vascular mass include enlarged pulmonary artery secondary to a large left-to-right intracardiac shunt; poststenotic dilatation of the pulmonary artery from pulmonary valve stenosis; tetralogy of Fallot with absent pulmonary valve; Eisenmenger’s complex; ductus aneurysm; pulmonary artery sling; patches following tetralogy repair; aneurysms caused by systemic-to-pulmonary artery shunts; aneurysms of the main pulmonary artery or right ventricular outflow tract; pulmonary artery dissection; distention of the pulmonary artery by thrombus, embolus, or tumor; and pulmonary artery aneurysms due to infective endocarditis (Table 176-4).
Most congenital anomalies causing a vascular mediastinal mass associated with the pulmonary arterial tree are related to abnormalities of the pulmonary valve or intracardiac shunts. Surgical systemic-to–pulmonary artery shunts and other surgical procedures such as a right ventricle–to–pulmonary artery conduit can also cause vascular masses related to the shunt, conduit, or pulmonary artery. Large left-to-right shunts from an atrial or ventricular septal defect will initially cause enlargement of the proximal main pulmonary artery. The chest radiograph will show an enlarged heart and enlarged main pulmonary artery as a bulge adjacent to the aortic knob, with obliteration of the aortopulmonary window, and will suggest the presence of increased pulmonary blood flow (Fig. 176-6). As the pulmonary vascular resistance increases, the amount of pulmonary blood flow diminishes and the lung fields become dark, with the proximal pulmonary artery remaining large (Eisenmenger’s complex).182
Patients with congenital pulmonary valvular stenosis may develop poststenotic dilatation or aneurysms of the main and left pulmonary arteries from the jet effect of blood on these vessels. Without an associated intracardiac shunt, pulmonary blood flow will be normal. The proximal left pulmonary artery may also become dilated. The preferential dilatation of the left pulmonary artery is probably related to the direction of the jet into this vessel, because it is a more direct continuation of the pulmonary trunk than is the right branch.
Absence of the pulmonary valve may occur as an isolated anomaly but is seen more frequently with tetralogy of Fallot. Patients present early in infancy with cyanosis and severe respiratory distress. The regurgitation across the pulmonary annulus causes massive enlargement of the right and left pulmonary arteries, compressing the right and left mainstem bronchi. The pulmonary trunk is large and, at fluoroscopy, pulsates vigorously.
In addition to mediastinal enlargement from the pulmonary arteries, collapse or hyperinflation of the lung may be seen as a result of bronchial compression. These infants require surgical intervention early in life.46
In addition to mediastinal enlargement from the pulmonary arteries, collapse or hyperinflation of the lung may be seen as a result of bronchial compression. These infants require surgical intervention early in life.46
Enlargement or aneurysms of the main pulmonary artery trunk or its branches can be caused by severe chronic pulmonary hypertension of diverse etiology (Fig. 176-7).65,73,84,144,146,150,168,170,184 Unilateral enlargement of the left or right pulmonary artery at the hilum can be caused by a large pulmonary embolism and may simulate a perihilar mass. Appropriate history along with a diminution of vascular markings in the ipsilateral lung suggest the diagnosis, which can be confirmed by CT angiography.
Pulmonary artery aneurysm and pulmonary artery dissection are rare complications of chronic pulmonary hypertension. Unlike dissection in systemic arteries, pulmonary artery dissection is usually lethal.58,91 Diagnosis of pulmonary artery aneurysm with dissection is most commonly made at autopsy in cases of sudden and unexpected death. In only 14% of patients, the diagnosis is made during life.58,125
Anomalous origin of the left pulmonary artery from the right pulmonary artery causes a “pulmonary artery sling” to be formed, which is the left pulmonary artery coursing around the right mainstem bronchus, between the trachea and esophagus, to the left lung (see Chapter 82, on vascular rings). These patients usually present early in life with respiratory distress. A chest radiograph classically shows hyperinflation of the right lung. A mediastinal mass is seen on the lateral chest radiograph between the trachea and esophagus. Barium swallow typically reveals a prominent indentation in the anterior wall of the esophagus immediately above the tracheal bifurcation. Diagnosis is currently made by echocardiography, contrast-enhanced CT scan, or MRI. The first successful surgical repair of pulmonary artery sling was performed by Potts and colleagues.133
Ductus Arteriosus
Aneurysms of the ductus arteriosus present as a rounded mass adjacent to the aortic knob. In the infantile form, children can present with asphyxia at birth.70 If the child survives long enough to have a chest radiograph, it will reveal an oval mass with a convex rounded edge projecting from the left superior mediastinum.19,80,108,154,157,163 (Fig. 176-8). The second type of ductal aneurysm occurs in childhood or early adult life. Aneurysmal enlargement produces respiratory distress due to airway compression or hoarseness due to recurrent laryngeal nerve compression. Long-standing cases may demonstrate progressive enlargement with mural calcification. Surgical excision is warranted owing to the risks of rupture, dissection, endocarditis, and late pulmonary hypertension.
Rare vasculitis—such as Behçet’s disease, the Hughes-Stovins syndrome, and Alagille syndrome—may present with pulmonary artery aneurysms (Fig. 176-9).
Vascular Masses as the Result of Previous Surgical Procedures
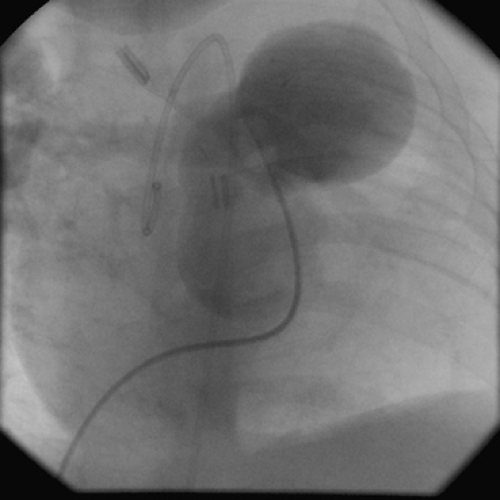
There are many causes of mediastinal “masses” following cardiac surgical procedures for congenital heart disease, particularly if conduits are used. A significant mediastinal mass can be formed by an aneurysm of the right ventricular infundibular patch following repair of tetralogy of Fallot. This occurs in about 1% of patients following tetralogy repair. It can stem from either a false aneurysm or thinning of the right ventricular patch, particularly if pericardium is used.143,148 This appears on plain chest radiographs as a progressive enlargement of the right ventricular border of the heart, especially on lateral views. Repair should be undertaken expeditiously to prevent risk of rupture. Patients with a right ventricle–to–pulmonary artery conduit can develop
a false aneurysm at the ventricular site on the distal anastomosis (Fig. 176-10).
a false aneurysm at the ventricular site on the distal anastomosis (Fig. 176-10).
Shunts from the systemic circulation to the pulmonary circulation as palliation for cyanotic heart disease with inadequate pulmonary blood flow can cause aneurysmal dilatation of the pulmonary artery if flow through the shunt is excessive. In particular, the Potts shunt, which was previously used in small babies with inadequate pulmonary blood flow, can cause massive pulmonary artery aneurysms (Fig. 176-11).134 In some cases the pulmonary artery calcifies, causing a spectacular appearance
on the chest radiograph (Fig. 176-12). Formation of a left pulmonary artery aneurysm is one of the reasons the Potts shunt is no longer used.
on the chest radiograph (Fig. 176-12). Formation of a left pulmonary artery aneurysm is one of the reasons the Potts shunt is no longer used.
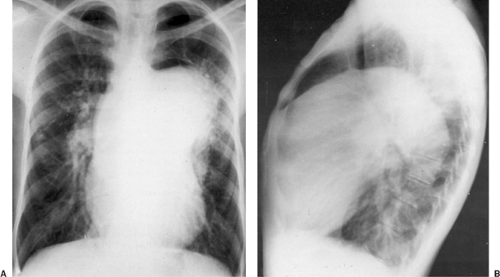 Figure 176-11. Chest radiographs (posteroanterior and lateral) of a 23-year-old patient with tetralogy of Fallot 16 years after a Potts (descending aorta–to–pulmonary artery) anastomosis. The left pulmonary artery aneurysm projects from the mediastinum into the left chest (A) and fills the anterior compartment (B).
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|